Which Elements Can Expand Their Octet
Muz Play
Mar 18, 2025 · 6 min read
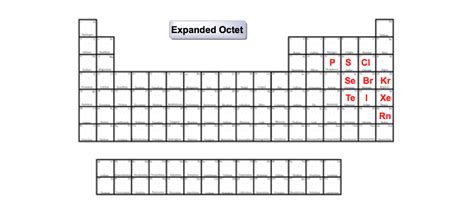
Table of Contents
Which Elements Can Expand Their Octet?
The octet rule, a cornerstone of introductory chemistry, states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons, resembling the stable electron configuration of noble gases. While a remarkably useful guideline, many elements routinely violate this rule. Understanding which elements can expand their octet and why is crucial for comprehending the behavior of numerous compounds and predicting their properties. This article delves into the exceptions to the octet rule, focusing on the elements capable of exceeding eight electrons in their valence shell.
Understanding the Octet Rule and its Limitations
The octet rule stems from the stability associated with a filled valence shell. Atoms achieve this stability by interacting with other atoms through ionic or covalent bonding. Ionic bonds involve the transfer of electrons, while covalent bonds involve the sharing of electrons. The octet rule effectively explains the bonding in a vast number of simple compounds.
However, the octet rule is not a rigid law; it's more of a helpful guideline with significant exceptions. Several factors influence whether an element adheres to or deviates from the octet rule:
-
Principal Quantum Number (n): Elements in the third period and beyond have access to d orbitals in their valence shell. These d orbitals can accommodate additional electrons, allowing for the expansion of the octet. Elements in the second period (like boron, carbon, nitrogen, oxygen, and fluorine) lack d orbitals, strictly limiting their ability to exceed eight electrons.
-
Electronegativity: The electronegativity of the central atom and the surrounding atoms influences bonding. Highly electronegative atoms are less likely to share electrons beyond the octet, preferring to maintain a stable electron configuration.
-
Formal Charge: Minimizing formal charge on all atoms is a key factor in determining the stability and preferred structure of a molecule. Expanding the octet can sometimes help reduce formal charges, leading to a more stable structure.
Elements that Commonly Expand Their Octet
The ability to expand the octet is primarily observed in elements from the third period onwards. These elements include:
-
Phosphorus (P): Phosphorus is a classic example. In phosphorus pentachloride (PCl₅), phosphorus has ten electrons in its valence shell (five bonds contributing ten electrons). The 3d orbitals are utilized to accommodate these extra electrons.
-
Sulfur (S): Sulfur hexafluoride (SF₆) is a highly stable compound where sulfur exhibits twelve valence electrons. This is possible due to the involvement of 3d orbitals.
-
Chlorine (Cl): Chlorine can also exceed the octet, as seen in compounds like chlorine trifluoride (ClF₃), where chlorine has ten valence electrons.
-
Iodine (I): Iodine, like chlorine, can expand its octet, particularly with highly electronegative atoms such as fluorine. Iodine pentafluoride (IF₅) and iodine heptafluoride (IF₇) are examples of iodine exceeding eight valence electrons.
Detailed Examples:
1. Phosphorus Pentachloride (PCl₅):
Phosphorus has five valence electrons. In PCl₅, it forms five covalent bonds with five chlorine atoms, resulting in ten electrons surrounding the phosphorus atom. This expansion of the octet is accommodated by the use of 3d orbitals. The Lewis structure shows five bonding pairs and no lone pairs on phosphorus.
2. Sulfur Hexafluoride (SF₆):
Sulfur has six valence electrons. In SF₆, it forms six covalent bonds with six fluorine atoms, leading to twelve valence electrons around sulfur. Again, the 3d orbitals participate in accommodating these extra electrons. The Lewis structure shows six bonding pairs and no lone pairs on sulfur.
3. Xenon Compounds:
While noble gases were once believed to be inert, the discovery of xenon compounds challenged this assumption. Xenon, with its large atomic size and readily available d orbitals, can form compounds like xenon tetrafluoride (XeF₄), where xenon exhibits twelve valence electrons.
Why Octet Expansion is Possible: The Role of d-Orbitals
The key to understanding octet expansion lies in the availability of empty d orbitals in the valence shell of the relevant atoms. Elements in the third period and beyond have access to 3d, 4d, 5d, and 6d orbitals. These orbitals are energetically close enough to the valence s and p orbitals to participate in bonding. The additional electrons needed to exceed the octet can occupy these empty d orbitals.
Factors Influencing Octet Expansion
While the presence of d orbitals is a necessary condition, it’s not sufficient alone. Other factors influence the likelihood of octet expansion:
-
Electronegativity of Ligands: Highly electronegative ligands, such as fluorine, can stabilize the expansion of the octet by effectively withdrawing electron density from the central atom. This reduces electron-electron repulsion and makes the expanded octet configuration more stable.
-
Size of Central Atom: Larger central atoms can more readily accommodate additional electrons because their valence electrons are further from the nucleus and experience less repulsion.
-
Steric Factors: The size and shape of the ligands can affect the stability of the expanded octet. Large ligands may cause steric hindrance, making octet expansion less favorable.
Molecules with Incomplete Octets
Conversely, some molecules exist where the central atom has fewer than eight electrons in its valence shell. This is known as an incomplete octet. This is primarily observed in elements from the second period (with fewer available orbitals), particularly boron and beryllium. These elements are often electron-deficient. Examples include:
-
Boron trifluoride (BF₃): Boron has only six electrons around it.
-
Beryllium chloride (BeCl₂): Beryllium has only four electrons around it.
These molecules are often highly reactive due to their incomplete octets and tendency to accept additional electron pairs.
Predicting Octet Expansion: A Practical Approach
While there's no single, foolproof method, several guidelines can help predict whether an element will expand its octet:
-
Identify the central atom: Determine the element around which the other atoms are bonded.
-
Check the period: If the central atom is from the third period or beyond, octet expansion is possible.
-
Consider the electronegativity of ligands: Highly electronegative ligands increase the likelihood of octet expansion.
-
Assess steric factors: Large ligands can hinder octet expansion.
-
Draw Lewis structures: Trying to draw a Lewis structure obeying the octet rule. If it's impossible without exceeding the octet, then expansion is likely.
Conclusion: Beyond the Octet Rule
The octet rule serves as an essential introduction to chemical bonding, but it's vital to remember its limitations. Many elements, particularly those in the third period and beyond, routinely expand their octet to achieve more stable configurations. Understanding the factors influencing octet expansion – the availability of d orbitals, electronegativity of ligands, and steric considerations – is crucial for correctly predicting the structures and properties of a wide range of compounds. The concept of octet expansion highlights the dynamic and nuanced nature of chemical bonding, reminding us that simple rules are often refined by a deeper understanding of atomic structure and electron behavior. Further exploration into advanced bonding theories provides even more comprehensive insights into these complex interactions.
Latest Posts
Latest Posts
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Elements Can Expand Their Octet . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
