Which Of The Following Is A Lewis Base
Muz Play
Mar 22, 2025 · 6 min read
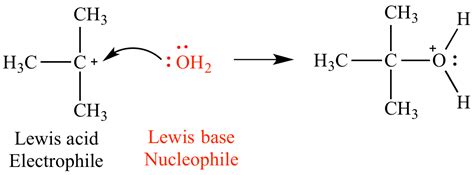
Table of Contents
Which of the Following is a Lewis Base? Understanding Lewis Acid-Base Theory
Lewis acid-base theory, a cornerstone of chemistry, provides a broader understanding of acid-base reactions than the traditional Brønsted-Lowry theory. While Brønsted-Lowry focuses on proton (H⁺) transfer, Lewis theory centers on the donation and acceptance of electron pairs. This significantly expands the scope of what we consider an acid or a base, leading to a richer understanding of chemical reactivity. This article delves deep into Lewis base definition, exploring its characteristics, examples, and its importance in various chemical processes. We'll also tackle how to identify a Lewis base amongst a selection of compounds.
Defining a Lewis Base: The Electron Pair Donor
A Lewis base is defined as a species that donates a pair of electrons to form a new covalent bond with a Lewis acid. The crucial aspect here is the donation of an electron pair. This electron pair resides in a lone pair of electrons on the Lewis base, often associated with a non-bonding orbital. This donated electron pair is then shared between the Lewis base and the Lewis acid, forming a coordinate covalent bond (also known as a dative bond).
Key Characteristics of Lewis Bases:
- Presence of Lone Pairs: The defining characteristic is the presence of one or more lone pairs of electrons. These lone pairs are readily available for donation to an electron-deficient species.
- Electron-Rich Species: Lewis bases are generally electron-rich species, meaning they have a higher electron density than the Lewis acid they interact with.
- Nucleophilic Nature: Many Lewis bases also exhibit nucleophilic behavior. Nucleophiles are species that donate electrons to electron-deficient atoms, often carbon atoms in organic chemistry. This overlap between Lewis bases and nucleophiles is significant and highlights the importance of electron donation in both concepts.
- Varied Strength: The strength of a Lewis base varies significantly depending on several factors, including the electronegativity of the atom donating the electron pair, the size of the atom, and steric hindrance.
Contrasting Lewis Bases with Brønsted-Lowry Bases
It's vital to understand the differences between Lewis and Brønsted-Lowry bases. While all Brønsted-Lowry bases are also Lewis bases, the reverse isn't true.
- Brønsted-Lowry Base: Donates a proton (H⁺). This definition is limited to species capable of accepting a proton.
- Lewis Base: Donates an electron pair. This definition encompasses a much wider range of species, including those that cannot accept a proton.
For example, ammonia (NH₃) acts as both a Brønsted-Lowry base (accepting a proton) and a Lewis base (donating its lone pair of electrons). However, consider a compound like trimethylamine (N(CH₃)₃). It acts as a Lewis base by donating its lone pair but cannot readily accept a proton due to the steric hindrance from the methyl groups. This illustrates the broader scope of the Lewis definition.
Examples of Lewis Bases: A Diverse Spectrum
The range of compounds that act as Lewis bases is vast, encompassing various classes of molecules and ions. Here are some prominent examples:
1. Ammonia (NH₃) and Amines (R-NH₂, R₂-NH, R₃-N):
Ammonia and amines are classic examples of Lewis bases. The nitrogen atom possesses a lone pair of electrons that can be donated to a Lewis acid. The strength of the base can vary depending on the substituents (R groups) attached to the nitrogen atom. Electron-donating groups increase base strength, while electron-withdrawing groups decrease it.
2. Water (H₂O):
Water, while often considered a Brønsted-Lowry base, is also a Lewis base due to the two lone pairs on the oxygen atom. This allows it to donate electrons to various Lewis acids. Water's role as a ligand in many transition metal complexes demonstrates this Lewis base behavior.
3. Halide Ions (F⁻, Cl⁻, Br⁻, I⁻):
Halide ions possess lone pairs and readily donate them to Lewis acids. The fluoride ion (F⁻) is a particularly strong Lewis base due to its high charge density and small size.
4. Alcohols (R-OH):
Similar to water, alcohols contain an oxygen atom with two lone pairs, making them Lewis bases. The strength varies based on the nature of the R group.
5. Ethers (R-O-R):
Ethers also possess an oxygen atom with two lone pairs capable of donation to Lewis acids.
6. Phosphines (R₃P):
Phosphines are analogous to amines but contain phosphorus instead of nitrogen. They are often stronger Lewis bases than amines.
7. Carbanions (R⁻):
Carbanions, negatively charged carbon atoms, are strong Lewis bases due to their high electron density and negative charge.
Identifying a Lewis Base: A Practical Approach
When faced with a series of compounds, identifying the Lewis base requires careful consideration of the electronic structure. Look for:
- Atoms with Lone Pairs: Focus on atoms with lone pairs of electrons, especially those from groups 15-17 in the periodic table (nitrogen, oxygen, phosphorus, sulfur, halogens).
- Negative Charge: Negatively charged species generally have excess electrons and are therefore strong Lewis bases.
- Electron-Donating Groups: If the atom with a lone pair is attached to electron-donating groups, the base strength increases.
- Steric Hindrance: Bulky substituents can hinder the ability of the lone pair to interact with a Lewis acid, decreasing the base strength.
Applications of Lewis Acid-Base Theory: Beyond the Basics
Lewis acid-base theory is not confined to simple reactions. Its applications extend to numerous areas of chemistry:
1. Catalysis:
Many catalysts operate through Lewis acid-base interactions. Lewis acids can activate substrates by accepting electron pairs, while Lewis bases can activate other reagents. This is crucial in organic synthesis and industrial processes.
2. Coordination Chemistry:
Coordination complexes are formed through the interaction of Lewis acids (metal ions) and Lewis bases (ligands). Understanding this interaction is fundamental to understanding the properties and reactivity of coordination compounds, which have wide applications in materials science and biological systems.
3. Biochemistry:
Numerous biological processes involve Lewis acid-base interactions. Enzyme catalysis, DNA structure, and metal ion binding in proteins all rely on the principles of Lewis acid-base theory.
4. Materials Science:
The design and synthesis of new materials often rely on controlled Lewis acid-base interactions. This is crucial in developing new catalysts, polymers, and other functional materials.
Conclusion: The Significance of Lewis Bases in Chemistry
Lewis base theory provides a crucial framework for understanding chemical reactivity across a vast array of systems. By understanding the principles of electron pair donation and the factors influencing the strength of Lewis bases, we can gain valuable insights into diverse chemical processes, from simple reactions to complex biochemical and materials science applications. The ability to identify Lewis bases is essential for any chemist, whether studying organic synthesis, inorganic complexes, or biochemical processes. The examples and guidelines presented here equip you with the tools necessary to confidently determine which compounds qualify as Lewis bases. Remember, the presence of readily available lone pairs is the defining factor.
Latest Posts
Latest Posts
-
Which Kingdom Includes Sea Anemones Snails Humans Insects And Birds
Mar 23, 2025
-
Lewis Structure Of Co With Formal Charges
Mar 23, 2025
-
What Are The Two Divisions Of The Skeleton
Mar 23, 2025
-
One Party Democratic Dominance Occurred From Reconstruction Until The
Mar 23, 2025
-
Vocabulario De Salon De Clases En Ingles
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Lewis Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
