Which Of The Following Is Polar Molecule
Muz Play
Mar 25, 2025 · 6 min read
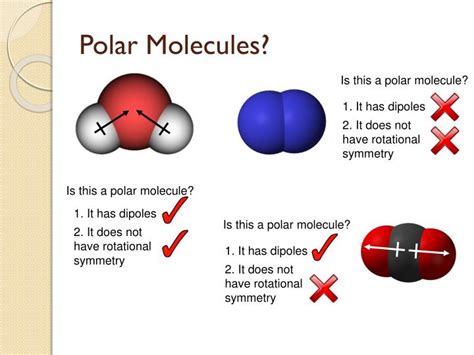
Table of Contents
Which of the Following is a Polar Molecule? Understanding Molecular Polarity
Determining whether a molecule is polar or nonpolar is a fundamental concept in chemistry with significant implications for various properties like solubility, boiling point, and reactivity. This comprehensive guide will delve deep into understanding molecular polarity, providing you with the tools to identify polar molecules effectively. We'll explore the concepts of electronegativity, bond polarity, molecular geometry, and dipole moments, and apply them to various examples.
Understanding Polarity: Electronegativity and Bond Dipoles
At the heart of molecular polarity lies the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Elements on the right side of the periodic table (excluding noble gases) generally have higher electronegativities than those on the left. Fluorine (F) is the most electronegative element.
When two atoms with different electronegativities form a bond, the electrons are not shared equally. The more electronegative atom pulls the electrons closer, creating a polar bond or a bond dipole. This unequal sharing of electrons results in a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom.
Example: In a hydrogen chloride (HCl) molecule, chlorine (Cl) is more electronegative than hydrogen (H). Therefore, the electrons in the H-Cl bond are pulled closer to the chlorine atom, making the chlorine atom partially negative (δ-) and the hydrogen atom partially positive (δ+).
Molecular Geometry and Dipole Moments
The presence of polar bonds doesn't automatically make a molecule polar. The overall polarity of a molecule depends on both the bond polarities and the molecular geometry. Molecular geometry describes the three-dimensional arrangement of atoms in a molecule.
A dipole moment (μ) is a vector quantity that represents the overall polarity of a molecule. It is the product of the magnitude of the charge separation and the distance between the charges. If the bond dipoles cancel each other out due to symmetry in the molecular geometry, the molecule is nonpolar, even if it contains polar bonds. If the bond dipoles do not cancel each other out, the molecule is polar.
Identifying Polar Molecules: A Step-by-Step Approach
To determine if a molecule is polar, follow these steps:
-
Draw the Lewis Structure: This helps visualize the arrangement of atoms and bonds.
-
Identify Bond Polarities: Determine the electronegativity difference between each pair of bonded atoms. A significant difference (generally greater than 0.4 on the Pauling scale) indicates a polar bond.
-
Determine Molecular Geometry: Use VSEPR (Valence Shell Electron Pair Repulsion) theory to predict the three-dimensional arrangement of atoms. Common geometries include linear, bent, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.
-
Analyze Dipole Cancellation: Determine whether the bond dipoles cancel each other out based on the molecular geometry. Symmetrical molecules often have nonpolar characteristics.
Examples: Polar vs. Nonpolar Molecules
Let's analyze some examples to solidify our understanding:
1. Carbon Dioxide (CO₂):
- Lewis Structure: O=C=O
- Bond Polarity: C=O bonds are polar because oxygen is more electronegative than carbon.
- Molecular Geometry: Linear
- Dipole Moment: The two C=O bond dipoles are equal in magnitude and point in opposite directions, resulting in a net dipole moment of zero. Therefore, CO₂ is a nonpolar molecule.
2. Water (H₂O):
- Lewis Structure: H-O-H
- Bond Polarity: O-H bonds are polar because oxygen is more electronegative than hydrogen.
- Molecular Geometry: Bent
- Dipole Moment: The two O-H bond dipoles do not cancel each other out due to the bent geometry, resulting in a net dipole moment. Therefore, H₂O is a polar molecule.
3. Methane (CH₄):
- Lewis Structure: A central carbon atom bonded to four hydrogen atoms.
- Bond Polarity: C-H bonds are considered slightly polar, though the electronegativity difference is small.
- Molecular Geometry: Tetrahedral
- Dipole Moment: The four C-H bond dipoles cancel each other out due to the symmetrical tetrahedral geometry. Therefore, CH₄ is generally considered a nonpolar molecule. The slight polarity of the individual bonds is negligible in the overall molecule.
4. Ammonia (NH₃):
- Lewis Structure: A central nitrogen atom bonded to three hydrogen atoms, with one lone pair of electrons.
- Bond Polarity: N-H bonds are polar due to the electronegativity difference between nitrogen and hydrogen.
- Molecular Geometry: Trigonal pyramidal
- Dipole Moment: The three N-H bond dipoles and the lone pair's influence combine to create a net dipole moment. Therefore, NH₃ is a polar molecule.
5. Carbon Tetrachloride (CCl₄):
- Lewis Structure: A central carbon atom bonded to four chlorine atoms.
- Bond Polarity: C-Cl bonds are polar.
- Molecular Geometry: Tetrahedral
- Dipole Moment: The four C-Cl bond dipoles cancel each other out due to the symmetrical tetrahedral geometry. Therefore, CCl₄ is a nonpolar molecule.
6. Chloroform (CHCl₃):
- Lewis Structure: A central carbon atom bonded to one hydrogen atom and three chlorine atoms.
- Bond Polarity: C-Cl bonds are polar, and the C-H bond is slightly polar.
- Molecular Geometry: Tetrahedral
- Dipole Moment: The C-Cl bond dipoles do not cancel out the effect of the C-H bond dipole, resulting in a net dipole moment. Therefore, CHCl₃ is a polar molecule.
Advanced Considerations: Factors Affecting Polarity
While electronegativity and molecular geometry are primary determinants, other factors can subtly influence a molecule's polarity:
-
Bond Order: Multiple bonds (double or triple) generally have higher bond polarity than single bonds.
-
Hybridization: The type of hybridization (sp, sp², sp³) can affect bond angles and hence the overall dipole moment.
-
Inductive Effects: The presence of electron-withdrawing or electron-donating groups in a molecule can affect the distribution of electron density and therefore the polarity.
Applications of Molecular Polarity
Understanding molecular polarity is crucial in several areas of chemistry and related fields:
-
Solubility: Polar molecules tend to dissolve in polar solvents (like water), while nonpolar molecules dissolve in nonpolar solvents (like oil). This is the basis of the "like dissolves like" rule.
-
Boiling Point: Polar molecules generally have higher boiling points than nonpolar molecules of comparable size due to stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding).
-
Spectroscopy: Molecular polarity influences the behavior of molecules in various spectroscopic techniques, such as infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy.
-
Biological Systems: Polarity plays a vital role in the structure and function of biomolecules like proteins and DNA, where hydrogen bonding between polar groups is essential.
Conclusion: Mastering Molecular Polarity
Determining whether a molecule is polar or nonpolar is a fundamental skill in chemistry. By understanding electronegativity, bond polarity, molecular geometry, and dipole moments, you can accurately predict the polarity of a vast range of molecules. This knowledge is essential for comprehending various chemical and physical properties, as well as for applications across multiple scientific disciplines. Remember to systematically analyze the Lewis structure, geometry, and bond dipoles to reach a definitive conclusion. The examples provided should serve as valuable tools for practicing and solidifying your understanding of this crucial concept.
Latest Posts
Latest Posts
-
The Membrane Holds The Coils Of The Small Intestine
Mar 28, 2025
-
Organelles In Eukaryotic Cells Answer Key
Mar 28, 2025
-
Lewis Dot Diagram For Ionic Bonding Between Li And F
Mar 28, 2025
-
What Is The Relationship Between Avogadros Number And The Mole
Mar 28, 2025
-
What Are The Building Blocks For Fats
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is Polar Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
