Which One Is Noble Gas Metallloids
Muz Play
Mar 20, 2025 · 5 min read
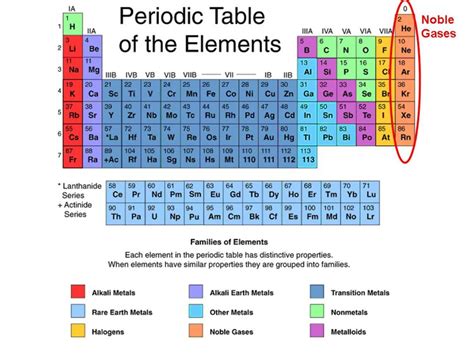
Table of Contents
Which One is a Noble Gas Metalloid? A Deep Dive into the Periodic Table
The question, "Which one is a noble gas metalloid?" might initially seem straightforward. However, the answer requires a nuanced understanding of the definitions of "noble gas," "metalloid," and the inherent limitations of such classifications. The simple answer is: there are no noble gas metalloids. This article delves into the reasons why, exploring the properties of noble gases and metalloids to clarify the fundamental incompatibility of these classifications.
Understanding Noble Gases
Noble gases, also known as inert gases, occupy Group 18 of the periodic table. This group includes Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn), and the synthetically produced Oganesson (Og). Their defining characteristic is their exceptional stability. This stems from their complete valence electron shells, meaning they have a full complement of electrons in their outermost energy level. This full octet (or duet for Helium) renders them extremely unreactive, hence their historical designation as "inert gases." While the term "inert" is somewhat outdated given the discovery of noble gas compounds, their inherent low reactivity remains a cornerstone of their identity.
Key properties of noble gases:
- Extremely low reactivity: Their full valence shells make them highly resistant to forming chemical bonds with other elements.
- Colorless, odorless, and tasteless: Under standard conditions, they exist as monatomic gases.
- Low boiling points: They have weak interatomic forces, leading to low boiling points.
- Poor conductors of electricity and heat: Their electronic structure contributes to their poor conductivity.
Understanding Metalloids
Metalloids, also known as semimetals, are elements that exhibit properties intermediate between those of metals and nonmetals. They occupy a diagonal band on the periodic table, separating the metals from the nonmetals. This intermediate nature results in a fascinating array of properties that make them crucial in various technological applications. Their position on the periodic table reflects their ambiguous nature, making their categorization more complex.
Key properties of metalloids:
- Variable conductivity: Their electrical conductivity is often sensitive to temperature and other external factors, exhibiting semiconducting behavior.
- Brittle solids: They are typically brittle and not easily deformed.
- Metallic luster (sometimes): Some metalloids display a metallic sheen, while others appear dull.
- Intermediate electronegativity: Their electronegativity values fall between those of metals and nonmetals.
- Ability to form both ionic and covalent bonds: This reflects their position between metals and nonmetals in the periodic table.
The Incompatibility of Noble Gases and Metalloids
The fundamental reason why there are no noble gas metalloids lies in their contrasting electronic configurations and resulting properties. Noble gases have complete valence electron shells, rendering them exceptionally stable and chemically inert. This inherent stability directly opposes the behavior of metalloids, which tend to participate in chemical bonding, albeit in a manner different from typical metals or nonmetals. The very nature of a metalloid requires a certain level of reactivity and willingness to share or transfer electrons, a property completely absent in noble gases.
Specific Contrasts:
- Reactivity: Noble gases are extremely unreactive, while metalloids exhibit intermediate reactivity.
- Electrical Conductivity: Noble gases are poor conductors, while metalloids demonstrate semiconducting behavior, exhibiting variable conductivity.
- Bonding: Noble gases rarely form bonds, while metalloids form both ionic and covalent bonds.
- Physical State: While several metalloids are solids at room temperature, noble gases are all gases.
To be classified as a metalloid, an element must exhibit some degree of metallic and non-metallic character. This necessitates the ability to form chemical bonds, a fundamental characteristic that is largely absent in noble gases. While some heavier noble gases (like Xenon) can form compounds under extreme conditions, this is an exception that proves the rule. Their exceptional stability and low reactivity firmly place them outside the realm of metalloid characteristics.
Exploring the Exceptions: The Case of Xenon Compounds
The synthesis of xenon compounds in the late 20th century challenged the long-held belief in the complete inertness of noble gases. Compounds such as xenon tetrafluoride (XeF₄) and xenon hexafluoroplatinate (Xe[PtF₆]) demonstrate that under specific, highly energetic conditions, xenon can participate in chemical bonding. However, even these exceptional cases do not qualify xenon (or any noble gas) as a metalloid. The formation of these compounds requires extreme conditions, and the resulting compounds are generally unstable. This behavior is fundamentally different from the characteristics exhibited by typical metalloids, which often display a range of stable compounds under more ordinary conditions.
Misconceptions and Clarifications
The confusion might stem from the periodic table's organization and the gradual transitions in properties across elements. While the properties of elements change gradually as you move across and down the periodic table, the leap from the exceptionally stable noble gases to the relatively reactive metalloids is significant. There is no overlap or intermediary state bridging these distinct groups.
It is crucial to understand that classifying elements is a simplification of complex behavior. While models like the periodic table provide a valuable framework for understanding chemical properties, they are not perfect representations of reality. The behavior of elements is often more nuanced and context-dependent than these simplified classifications suggest.
Conclusion: The Absence of Noble Gas Metalloids
In summary, there are no noble gas metalloids. The fundamental properties of noble gases—their exceptional stability due to complete valence electron shells and their extremely low reactivity—directly contradict the intermediate characteristics of metalloids, which exhibit variable conductivity, participate in chemical bonding, and display a mixture of metallic and non-metallic properties. While the discovery of noble gas compounds has expanded our understanding of their reactivity, these exceptions do not alter their fundamental incompatibility with the definition of a metalloid. The clear distinction between these two groups remains a cornerstone of our understanding of the periodic table and the behavior of elements.
Latest Posts
Latest Posts
-
Is Viscosity A Physical Or Chemical Property
Mar 21, 2025
-
How Do You Calculate The Rate Of Diffusion
Mar 21, 2025
-
What Is A Unique Property Of Water
Mar 21, 2025
-
In A Phospholipid Molecule The Head Is
Mar 21, 2025
-
How To Find The Coefficient Chemistry Calculus
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Which One Is Noble Gas Metallloids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
