Is Viscosity A Physical Or Chemical Property
Muz Play
Mar 21, 2025 · 5 min read
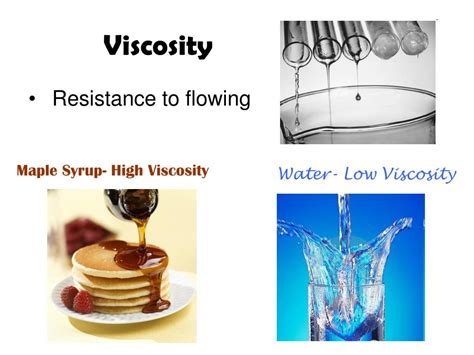
Table of Contents
Is Viscosity a Physical or Chemical Property? A Deep Dive
Viscosity, a measure of a fluid's resistance to flow, is a concept crucial across numerous scientific disciplines, from engineering and physics to chemistry and biology. Understanding whether viscosity is a physical or chemical property is fundamental to grasping its behavior and applications. While the answer might seem straightforward, a deeper exploration reveals a nuanced perspective. This article will delve into the intricacies of viscosity, differentiating physical from chemical properties and ultimately clarifying its classification.
Understanding Viscosity: A Fundamental Overview
Before diving into the classification debate, let's establish a solid understanding of viscosity itself. Viscosity quantifies a fluid's resistance to deformation under shear stress or tensile stress. Imagine pouring honey versus water; honey flows much slower due to its higher viscosity. This resistance stems from the internal friction between the fluid's molecules. The stronger the intermolecular forces, the greater the viscosity.
Several factors influence a fluid's viscosity:
-
Temperature: Generally, viscosity decreases with increasing temperature. Higher temperatures provide molecules with more kinetic energy, allowing them to overcome intermolecular forces and flow more freely. This is why honey flows more easily when warmed.
-
Pressure: Pressure's impact on viscosity is more complex and often dependent on the fluid's nature. In many liquids, increased pressure leads to slightly higher viscosity.
-
Molecular Structure: The shape and size of molecules significantly affect viscosity. Larger, more complex molecules tend to exhibit higher viscosity due to increased intermolecular interactions.
-
Intermolecular Forces: The strength of forces like van der Waals forces, hydrogen bonds, and dipole-dipole interactions directly impacts viscosity. Stronger forces lead to greater resistance to flow.
-
Concentration: For solutions and mixtures, the concentration of solute significantly influences viscosity. Higher concentrations often result in higher viscosity.
Defining Physical and Chemical Properties
To accurately classify viscosity, we need precise definitions of physical and chemical properties:
Physical Properties: These are characteristics that can be observed or measured without changing the substance's chemical composition. Examples include color, density, melting point, boiling point, and viscosity. Measuring these properties does not alter the fundamental chemical structure of the material.
Chemical Properties: These describe how a substance reacts or changes when interacting with other substances. They reveal information about a substance's reactivity. Examples include flammability, reactivity with acids, and oxidation potential. Observing chemical properties inherently alters the substance's chemical composition.
Why Viscosity is Considered a Physical Property
The key to understanding viscosity's classification lies in the fact that measuring viscosity doesn't change the fluid's chemical composition. We can measure the viscosity of a liquid using various techniques (e.g., viscometers) without altering its molecular structure or creating new substances. The fluid remains chemically identical before and after the measurement. This aligns perfectly with the definition of a physical property.
Furthermore, factors influencing viscosity, like temperature and pressure, primarily affect the kinetic energy and intermolecular spacing of the molecules, not their fundamental chemical bonds or structure. Changing the temperature of water alters its viscosity but doesn't transform it into a different substance.
The Nuances: Context Matters
While the general consensus classifies viscosity as a physical property, subtle nuances arise in specific contexts:
Non-Newtonian Fluids: These fluids exhibit viscosity that changes with applied shear stress or shear rate. Examples include ketchup, blood, and many polymer solutions. While the underlying chemical composition remains unchanged, the apparent viscosity changes depending on the conditions. This apparent change might seem to blur the lines, but the fundamental chemical structure doesn't transform. The behavior is still a consequence of physical interactions, even if complex ones.
Temperature-Dependent Viscosity and Chemical Changes: While a change in temperature primarily impacts the kinetic energy of molecules, extremely high temperatures can induce chemical decomposition or structural changes in certain fluids, thus altering their viscosity. This is an exceptional case where a change in temperature indirectly influences the chemical composition. However, the initial viscosity measurement itself doesn't cause the chemical change.
Polymer Solutions and Viscosity: The viscosity of polymer solutions is strongly influenced by the polymer's molecular weight, chain entanglement, and interactions with the solvent. Modifying the polymer through chemical reactions (e.g., crosslinking) would undeniably alter its viscosity, impacting both physical and chemical properties. However, measuring the viscosity of an unmodified polymer solution is still observing a physical property.
Practical Applications of Understanding Viscosity's Nature
Classifying viscosity as a physical property has significant practical implications across numerous fields:
Fluid Mechanics and Engineering: Understanding viscosity is critical for designing pipelines, pumps, and other fluid handling systems. Engineers use viscosity data to predict fluid flow behavior, optimize designs, and ensure efficient operation.
Material Science: Viscosity plays a key role in the processing and properties of materials like polymers, paints, and adhesives. Controlling viscosity is essential to achieve the desired consistency and performance characteristics.
Food Science: The viscosity of food products is crucial for texture, mouthfeel, and consumer acceptance. Food scientists leverage viscosity measurements to ensure product quality and consistency.
Biomedical Engineering: Blood viscosity is a critical factor in cardiovascular health. Understanding and monitoring blood viscosity aids in diagnosing and treating various cardiovascular diseases.
Environmental Science: Viscosity affects the transport and fate of pollutants in the environment, influencing their dispersion and potential impacts on ecosystems.
Conclusion: A Definitive Answer with Caveats
In conclusion, viscosity is unequivocally classified as a physical property. Measuring viscosity does not change the chemical composition of the substance; it simply quantifies its resistance to flow, a characteristic determined by intermolecular forces and molecular structure. While complexities arise with non-Newtonian fluids and extreme temperature conditions, the fundamental principle remains unchanged. The apparent changes in viscosity under such conditions are still rooted in physical interactions, not chemical transformations. Understanding this distinction is fundamental for numerous scientific and engineering disciplines, guiding advancements in various fields. While temperature changes can indirectly lead to chemical changes at extreme levels, the direct measurement of viscosity itself remains a physical observation.
Latest Posts
Latest Posts
-
A Hydrocarbon Is Any Compound That Contains
Mar 28, 2025
-
In Order To Break A Bond Energy Must Be
Mar 28, 2025
-
How Do You Divide A Square Root
Mar 28, 2025
-
Proof Of Derivative Of Inverse Trig Functions
Mar 28, 2025
-
Boundary Between The Crust And Mantle
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Is Viscosity A Physical Or Chemical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
