Which One Of These Is An Amino Group
Muz Play
Mar 21, 2025 · 6 min read
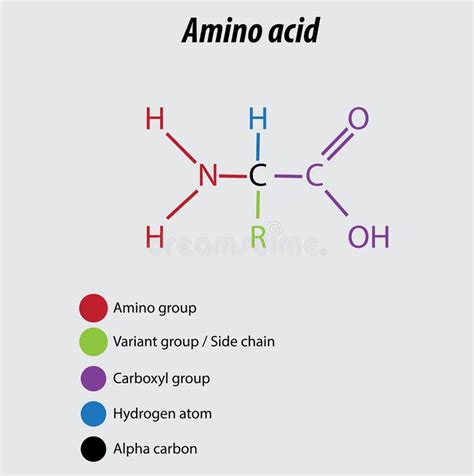
Table of Contents
Which One of These is an Amino Group? A Deep Dive into Organic Chemistry
Understanding functional groups is fundamental to organic chemistry. Among the many crucial functional groups, the amino group holds a significant place, playing a vital role in the structure and function of amino acids, proteins, and numerous other biologically active molecules. This comprehensive guide will delve into the intricacies of amino groups, explaining their structure, properties, and how to identify them within a molecule. We'll explore common misconceptions and provide you with the tools to confidently distinguish an amino group from other similar functional groups.
Understanding Functional Groups: The Building Blocks of Organic Molecules
Organic molecules, the cornerstone of life, are incredibly diverse. However, their complexity arises from the arrangement and combination of relatively few fundamental building blocks called functional groups. These groups are specific atoms or groups of atoms within a molecule that are responsible for its characteristic chemical reactions. Identifying these functional groups is crucial for predicting a molecule's properties and reactivity.
Key Functional Groups to Know
Before we focus on the amino group, let's briefly review some other important functional groups to establish a comparative understanding:
- Hydroxyl Group (-OH): Found in alcohols, this group is polar and capable of hydrogen bonding.
- Carbonyl Group (C=O): Present in aldehydes, ketones, carboxylic acids, and amides, this group is also polar and contributes to a molecule's reactivity.
- Carboxyl Group (-COOH): A combination of a carbonyl and a hydroxyl group, it's found in carboxylic acids, and is acidic.
- Amino Group (-NH₂): The star of our article, we will explore this group in detail in the following sections.
- Ether Group (-O-): A simple oxygen atom linking two carbon atoms.
- Ester Group (-COO-): Derived from carboxylic acids, this group is commonly found in fats and oils.
- Amide Group (-CONH₂): Related to carboxylic acids and amines, this group is crucial in peptide bonds.
Defining the Amino Group: Structure and Properties
The amino group, denoted as -NH₂, consists of a nitrogen atom bonded to two hydrogen atoms. This nitrogen atom possesses a lone pair of electrons, which makes the amino group a strong base. This lone pair readily accepts protons (H⁺), resulting in the formation of an ammonium ion (-NH₃⁺).
Key Characteristics of an Amino Group:
- Basic Nature: Due to the lone pair on nitrogen, amino groups readily accept protons, increasing the pH of a solution.
- Polarity: The presence of the nitrogen and hydrogen atoms creates a significant dipole moment, making the amino group highly polar. This polarity affects the solubility and other properties of the molecule containing the amino group.
- Hydrogen Bonding: The nitrogen atom in an amino group can participate in hydrogen bonding, influencing the structure and properties of molecules, particularly in biological systems. The ability to form hydrogen bonds is crucial for the stability of proteins and nucleic acids.
- Reactivity: The lone pair of electrons on the nitrogen makes the amino group highly reactive. It can undergo various reactions such as alkylation, acylation, and diazotization.
Identifying an Amino Group in a Molecule
Identifying an amino group within a larger molecule requires careful observation. Look for a nitrogen atom directly bonded to two hydrogen atoms (-NH₂). This simple structural feature is the hallmark of an amino group. However, it's essential to understand that the amino group can also be part of a larger structure. For example, it can be attached to a carbon atom within a carbon chain, or it can be part of a ring system.
Examples:
Let's illustrate this with a few examples:
- Methylamine (CH₃NH₂): In this simple molecule, the amino group is directly attached to a methyl group. The nitrogen atom has a lone pair of electrons and is bonded to two hydrogen atoms and one carbon atom.
- Glycine (NH₂CH₂COOH): This is the simplest amino acid, containing both an amino group (-NH₂) and a carboxyl group (-COOH). The amino group is bonded to the alpha carbon atom.
- Aniline (C₆H₅NH₂): Here, the amino group is attached to a benzene ring.
Distinguishing the Amino Group from Other Similar Functional Groups
Several functional groups share structural similarities with the amino group, but careful examination will reveal key differences:
- Amide Group (-CONH₂): While both contain nitrogen and hydrogen, the amide group also includes a carbonyl group (C=O) directly bonded to the nitrogen. This carbonyl group is absent in a simple amino group.
- Imine Group (C=N-): Imines have a carbon-nitrogen double bond. This double bond distinguishes them from amino groups with a single nitrogen-carbon bond.
- Nitro Group (-NO₂): This group contains a nitrogen atom double bonded to two oxygen atoms. It's distinctly different from the amino group in both its structure and properties.
The Importance of Amino Groups in Biological Systems
The amino group plays a critical role in the structure and function of numerous biomolecules:
Amino Acids and Proteins
Amino acids, the building blocks of proteins, all contain an amino group and a carboxyl group. The amino group's basic nature allows it to participate in peptide bond formation, linking amino acids together to form polypeptide chains. These chains then fold into complex three-dimensional structures that define the protein's function.
Nucleic Acids
Although not as directly involved as in proteins, amino groups are present in the nitrogenous bases of nucleic acids (DNA and RNA). These bases contribute to the genetic code and the intricate mechanisms of heredity.
Neurotransmitters
Several neurotransmitters, the chemical messengers in the nervous system, contain amino groups. For example, dopamine, norepinephrine, and serotonin all possess amino groups, influencing mood, sleep, and other vital functions.
Other Biological Roles
Amino groups are also found in various other biologically important molecules, such as:
- Certain vitamins: Some vitamins, such as vitamin B12, contain amino groups as part of their structure.
- Hormones: Some hormones, such as epinephrine (adrenaline), contain amino groups.
- Enzymes: Amino groups in enzyme active sites contribute to their catalytic functions.
Common Misconceptions about Amino Groups
It's important to clarify some common misconceptions surrounding amino groups:
- Amino group is always basic: While the amino group is generally basic, its basicity can be influenced by the surrounding chemical environment. For example, the basicity of the amino group in an amino acid can be affected by the presence of other functional groups in the molecule.
- Amino groups are only found in proteins: While amino groups are central to protein structure, they are found in a broad range of biologically active molecules, as detailed above.
- All nitrogen-containing groups are amino groups: This is incorrect. Many other nitrogen-containing groups exist, such as amides, nitriles, and nitro groups, each with distinct structures and properties.
Conclusion: Mastering Amino Group Identification
Understanding the amino group is crucial for grasping the complexities of organic chemistry and biochemistry. By understanding its structure, properties, and the subtle differences that distinguish it from similar functional groups, you can confidently identify it within any molecule. This knowledge is essential for comprehending the structure and function of biomolecules and for advancing in the fields of chemistry and biology. Remember to look for the characteristic -NH₂ group, acknowledging its basic nature and its capacity for hydrogen bonding and varied reactivity to properly identify and distinguish it in complex molecular structures. This understanding serves as a foundation for further exploration into the fascinating world of organic chemistry and its vital role in all living systems.
Latest Posts
Latest Posts
-
Types Of Chemical Reactions Answer Key
Mar 21, 2025
-
Metallic Character Of Elements In Periodic Table
Mar 21, 2025
-
What Is The Serial Position Curve
Mar 21, 2025
-
Label The Microscopic Anatomy Of Cardiac Muscle
Mar 21, 2025
-
Definition Of A Model In Psychology
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Which One Of These Is An Amino Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
