Which Particles Determine The Atomic Number Of An Element
Muz Play
Mar 26, 2025 · 5 min read
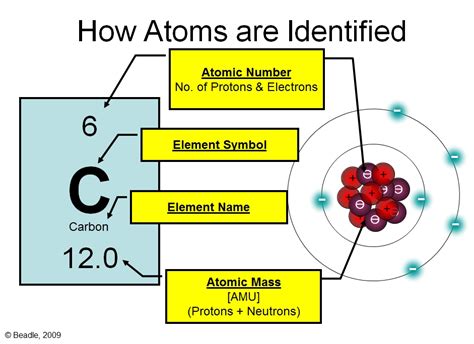
Table of Contents
Which Particles Determine the Atomic Number of an Element?
The atomic number of an element is a fundamental property that defines its identity and distinguishes it from all other elements. Understanding which particles determine this crucial number is key to grasping the very foundation of chemistry and physics. This comprehensive article will delve into the intricacies of atomic structure, exploring the role of protons, neutrons, and electrons in defining an element's atomic number and its implications for chemical behavior.
The Atomic Nucleus: The Heart of the Matter
Atoms, the basic building blocks of matter, are composed of three fundamental subatomic particles: protons, neutrons, and electrons. The vast majority of an atom's mass is concentrated in its nucleus, a tiny, dense region at the atom's center. This nucleus is where we find the protons and neutrons, collectively known as nucleons.
Protons: The Defining Particle
The atomic number of an element is determined solely by the number of protons found in its nucleus. This is a cornerstone principle of chemistry and physics. Each element has a unique and unchanging number of protons; it's like a fingerprint that identifies the element. For example, hydrogen (H) has one proton, helium (He) has two, lithium (Li) has three, and so on. This number remains constant regardless of the atom's state (solid, liquid, gas) or its chemical environment.
Key takeaway: The number of protons dictates the element's identity. If you change the number of protons, you fundamentally change the element itself.
Neutrons: Modifying Isotopes
Neutrons, while residing in the nucleus alongside protons, do not determine the atomic number. Instead, they contribute to an atom's mass number (the sum of protons and neutrons). Atoms of the same element can have different numbers of neutrons; these are called isotopes. Isotopes of an element have the same atomic number (same number of protons) but different mass numbers (different number of neutrons).
For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are carbon atoms because they both have six protons, but they differ in their mass numbers and are therefore isotopes. The different numbers of neutrons affect the isotope's stability and can lead to radioactive decay in certain cases.
Key takeaway: Neutrons influence the atom's mass and stability but not its identity as a specific element.
Electrons: Occupying the Orbitals
Electrons are negatively charged particles that orbit the nucleus in specific energy levels or shells. The number of electrons in a neutral atom is equal to the number of protons, ensuring a balanced electrical charge. However, electrons do not determine the atomic number. While they play a critical role in chemical bonding and reactivity, they are not fundamental in defining the element's identity.
Atoms can gain or lose electrons, forming ions (charged atoms). For example, a sodium atom (Na) can lose one electron to become a sodium ion (Na⁺), but it remains a sodium atom because it still has 11 protons in its nucleus. The change in electron number affects the atom's charge and its chemical behavior but not its atomic number.
Key takeaway: Electrons determine an atom's chemical properties and its ability to form bonds, but they don't define its elemental identity.
The Periodic Table: A Visual Representation
The periodic table of elements is a powerful tool that organizes elements based on their atomic number and recurring chemical properties. Elements are arranged in order of increasing atomic number, reflecting the systematic increase in the number of protons in their nuclei. The table's structure reveals patterns and trends in the properties of elements, allowing us to predict their behavior and understand their interactions.
The periodic table groups elements with similar chemical properties into columns (groups or families), highlighting the significance of the electron arrangement (and therefore, indirectly the number of protons) in determining chemical behavior. Rows (periods) represent elements with the same number of electron shells.
Implications of Atomic Number
The atomic number is not just a simple numerical identifier; it has profound implications across various fields:
-
Chemical Properties: The number of protons (and thus electrons in a neutral atom) determines an element's chemical behavior. The arrangement of electrons in shells and subshells governs how an element interacts with other elements, forming chemical bonds and compounds.
-
Nuclear Stability: The ratio of protons to neutrons in an atom's nucleus influences its stability. Certain combinations lead to stable isotopes, while others result in radioactive isotopes that undergo decay.
-
Spectroscopy: Each element emits and absorbs electromagnetic radiation at specific wavelengths, unique to its atomic number. Spectroscopic analysis is a powerful technique for identifying elements based on their spectral fingerprints.
-
Nuclear Reactions: Nuclear reactions involve changes in the nucleus of an atom, altering the number of protons or neutrons. Nuclear fission and fusion are prime examples, where the atomic number can change dramatically.
-
Material Science: The atomic number is crucial in understanding the properties of materials. The arrangement of atoms and their interactions, dictated by their atomic numbers, influence the material's strength, conductivity, and other properties.
Beyond the Basics: Exploring Isotopes and Nuclear Chemistry
While the atomic number defines the element, the existence of isotopes adds another layer of complexity. Isotopes have different numbers of neutrons, leading to variations in mass and stability. This has significant implications in various fields, including:
-
Radioactive Dating: The decay of specific radioactive isotopes (e.g., carbon-14) allows scientists to date ancient artifacts and geological formations.
-
Medical Imaging and Therapy: Radioactive isotopes are used in medical imaging techniques (PET scans) and radiation therapy to treat cancer.
-
Nuclear Power: Nuclear power plants utilize the controlled fission of isotopes like uranium-235 to generate electricity.
-
Nuclear Weapons: Nuclear weapons rely on uncontrolled nuclear reactions involving isotopes with high instability.
Conclusion
The atomic number, determined solely by the number of protons in an atom's nucleus, is the fundamental identifier of an element. It dictates the element's identity, chemical properties, and plays a critical role in nuclear stability and various applications across numerous scientific disciplines. While neutrons influence an atom's mass and stability (manifesting as isotopes), and electrons determine its chemical reactivity, it is the unwavering number of protons that definitively defines which element an atom represents. A deep understanding of the atomic number and its implications is paramount for anyone seeking a comprehensive grasp of the nature of matter and the world around us.
Latest Posts
Latest Posts
-
Why Does Electron Affinity Decrease Down A Group
Mar 29, 2025
-
How Many Valence Electrons Does Alkali Metals Have
Mar 29, 2025
-
The Functional Unit Of The Kidney Is Called The
Mar 29, 2025
-
Convert The Following Equation To Polar Coordinates
Mar 29, 2025
-
Calculate The Ph Of A Salt Solution
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Which Particles Determine The Atomic Number Of An Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
