Why Does Electron Affinity Decrease Down A Group
Muz Play
Mar 29, 2025 · 5 min read
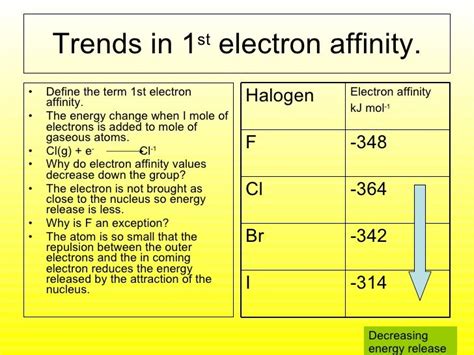
Table of Contents
Why Does Electron Affinity Decrease Down a Group? A Deep Dive into Periodic Trends
Electron affinity, a crucial concept in chemistry, describes the energy change that occurs when an atom gains an electron. Understanding its trends within the periodic table is vital for predicting chemical behavior and reactivity. One particularly intriguing trend is the general decrease in electron affinity down a group. While seemingly straightforward, the reasons behind this decrease are multifaceted and require a deeper understanding of atomic structure and electron-electron interactions. This article will delve into the complexities of this trend, explaining the contributing factors and exploring exceptions to the rule.
The Basics: What is Electron Affinity?
Before delving into the reasons for the decrease, let's establish a firm understanding of electron affinity itself. Electron affinity (Ea) is defined as the energy change associated with the addition of an electron to a neutral gaseous atom. A negative electron affinity value indicates that energy is released when an electron is added – a favorable exothermic process. Conversely, a positive electron affinity value indicates that energy is required to add an electron – an unfavorable endothermic process. In simpler terms, a highly negative electron affinity signifies a strong tendency for an atom to accept an electron.
It's crucial to remember that we're talking about gaseous atoms. The presence of other atoms or molecules significantly alters the energy landscape and complicates the measurement of electron affinity.
The Downward Trend: Why Electron Affinity Decreases Down a Group
The general trend of decreasing electron affinity down a group is primarily attributed to two interconnected factors:
1. Increased Atomic Size and Shielding Effect
As we move down a group in the periodic table, the atomic radius increases. This is because each successive element adds another electron shell, pushing the outermost electrons further away from the nucleus. This increased distance reduces the attractive force between the nucleus and the incoming electron. The added electrons in inner shells also provide a shielding effect, partially blocking the positive charge of the nucleus from the outermost electrons. This shielding effect further diminishes the attractive force, making it less energetically favorable for the atom to accept an additional electron.
Think of it like this: imagine trying to attract a small ball (the incoming electron) to a magnet (the nucleus). If you place the magnet further away, the attraction weakens. Adding layers of material between the magnet and the ball (shielding) further reduces the attraction. This analogy effectively illustrates the effect of increased atomic size and shielding on electron affinity.
2. Increased Electron-Electron Repulsion
Another significant factor is the increased electron-electron repulsion. As the atom gets larger and adds more electrons, the repulsion between the existing electrons and the incoming electron becomes increasingly significant. This repulsion opposes the attractive force of the nucleus, making it harder to add an electron and thus reducing the electron affinity. The negative charge of the already present electrons repels the newly added electron, lessening the overall attraction to the positively charged nucleus. This effect is particularly pronounced in larger atoms with many electrons.
Exceptions to the Rule: Why Some Elements Don't Follow the Trend
While the general trend is a decrease in electron affinity down a group, there are notable exceptions. These deviations are often due to specific electronic configurations and the stability associated with them. For instance:
-
Group 17 (Halogens): While the electron affinity generally decreases down this group, the decrease is not perfectly monotonic. This is partly due to the relatively small size of fluorine compared to the other halogens. Although fluorine has a strong nuclear charge, its small size leads to significant electron-electron repulsion, making its electron affinity slightly lower than chlorine's. Chlorine benefits from less electron-electron repulsion due to its larger size and consequently has a more negative electron affinity.
-
Group 14 (Carbon Group): This group exhibits an irregular trend as well. The electron affinity of carbon is relatively small (and even positive in some measurements). This is primarily due to the already half-filled p orbitals. The incoming electron would have to pair with an existing electron, resulting in significant electron-electron repulsion. The trend becomes more regular further down the group.
-
Noble Gases: The noble gases have exceptionally high ionization energies because they have a full outer shell. This means they are remarkably stable and show very low electron affinities. Adding an electron would require placing it in a higher energy level, requiring significant energy input.
These exceptions highlight the complexity of electron affinity and emphasize the importance of considering other factors beyond just atomic size and shielding.
The Role of Effective Nuclear Charge
The concept of effective nuclear charge (Z<sub>eff</sub>) is crucial in understanding electron affinity trends. Z<sub>eff</sub> represents the net positive charge experienced by an electron in an atom. It's less than the actual nuclear charge (Z) due to the shielding effect of inner electrons. As we move down a group, the nuclear charge increases, but the shielding effect increases even more significantly. This results in a relatively smaller increase in Z<sub>eff</sub>, leading to the weaker attraction for the incoming electron and therefore the lower electron affinity.
Practical Implications and Applications
Understanding the trends in electron affinity has many practical implications in various fields:
-
Predicting Chemical Reactivity: Elements with high electron affinities readily accept electrons, making them strong oxidizing agents. This knowledge is fundamental in predicting the outcome of chemical reactions.
-
Material Science: Electron affinity plays a vital role in determining the electronic properties of materials. This understanding is essential in designing new materials with specific electrical and optical characteristics.
-
Catalysis: The ability of a catalyst to accept or donate electrons is directly linked to its electron affinity, making this property essential in catalytic processes.
-
Environmental Science: Understanding electron affinity helps in predicting the behavior of pollutants and designing strategies for environmental remediation.
Conclusion
The decrease in electron affinity down a group is a complex interplay of several factors, primarily increased atomic size, enhanced shielding effect, and stronger electron-electron repulsion. While a general trend exists, exceptions highlight the nuances of atomic structure and electronic configurations. Understanding these factors is crucial for predicting chemical reactivity, designing new materials, and advancing our knowledge across various scientific disciplines. By grasping the underlying principles, we can better appreciate the intricate relationships between atomic properties and macroscopic behavior. The study of electron affinity and its periodic trends continues to be a fertile ground for scientific exploration and discovery.
Latest Posts
Latest Posts
-
What Type Of Chemical Reaction Is Represented By The Equation
Apr 01, 2025
-
Why Does Noncompetitive Inhibition Not Affect Km
Apr 01, 2025
-
What Are The Factors Affecting The Rate Of Diffusion
Apr 01, 2025
-
What Is The Vsepr Geometry Of The Particle
Apr 01, 2025
-
How To Calculate Current Through Each Resistor
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Why Does Electron Affinity Decrease Down A Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
