Why Does Noncompetitive Inhibition Not Affect Km
Muz Play
Apr 01, 2025 · 6 min read
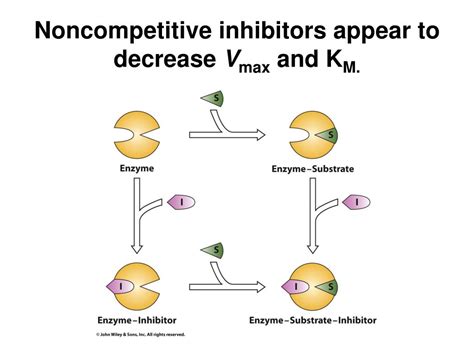
Table of Contents
Why Noncompetitive Inhibition Doesn't Affect K<sub>m</sub>: A Deep Dive into Enzyme Kinetics
Enzyme kinetics is a fundamental aspect of biochemistry, providing insights into the mechanisms of enzyme-catalyzed reactions and their regulation. Understanding enzyme inhibition is crucial, as it plays a significant role in various biological processes and therapeutic interventions. One type of inhibition, noncompetitive inhibition, presents a unique characteristic: it doesn't alter the Michaelis constant (K<sub>m</sub>). This article delves into the intricacies of noncompetitive inhibition, explaining why it leaves K<sub>m</sub> unchanged while affecting the maximum reaction velocity (V<sub>max</sub>).
Understanding Enzyme Kinetics and the Michaelis-Menten Equation
Before exploring noncompetitive inhibition, let's revisit the basics of enzyme kinetics and the Michaelis-Menten equation. This equation describes the relationship between the initial reaction velocity (v<sub>0</sub>), the substrate concentration ([S]), the maximum reaction velocity (V<sub>max</sub>), and the Michaelis constant (K<sub>m</sub>):
v<sub>0</sub> = (V<sub>max</sub>[S]) / (K<sub>m</sub> + [S])
-
V<sub>max</sub>: The maximum rate of the reaction when the enzyme is saturated with substrate. It represents the turnover number of the enzyme multiplied by the total enzyme concentration.
-
K<sub>m</sub>: The Michaelis constant. It represents the substrate concentration at which the reaction velocity is half of V<sub>max</sub> (v<sub>0</sub> = V<sub>max</sub>/2). K<sub>m</sub> is an indicator of the enzyme's affinity for its substrate; a lower K<sub>m</sub> indicates higher affinity.
Types of Enzyme Inhibition
Enzyme inhibitors are molecules that decrease the rate of enzyme-catalyzed reactions. They can be broadly classified into reversible and irreversible inhibitors. Reversible inhibitors can be further categorized into:
-
Competitive Inhibition: The inhibitor competes with the substrate for binding to the enzyme's active site. This increases the apparent K<sub>m</sub> (lower affinity) but doesn't affect V<sub>max</sub>.
-
Noncompetitive Inhibition: The inhibitor binds to an allosteric site (a site other than the active site) on the enzyme. This binding changes the enzyme's conformation, affecting its catalytic activity.
-
Uncompetitive Inhibition: The inhibitor binds only to the enzyme-substrate complex. This decreases both V<sub>max</sub> and K<sub>m</sub> proportionally.
-
Mixed Inhibition: The inhibitor can bind to both the free enzyme and the enzyme-substrate complex, but with different affinities. This type of inhibition affects both V<sub>max</sub> and K<sub>m</sub>, but not proportionally.
The Mechanism of Noncompetitive Inhibition and its Impact on K<sub>m</sub>
The key to understanding why noncompetitive inhibition doesn't affect K<sub>m</sub> lies in its mechanism of action. In noncompetitive inhibition, the inhibitor binds to an allosteric site on the enzyme. This binding induces a conformational change in the enzyme, altering the active site's shape and reducing its catalytic efficiency. Importantly, this conformational change affects the enzyme whether or not the substrate is bound.
This is fundamentally different from competitive inhibition. In competitive inhibition, the inhibitor only affects the enzyme when the substrate is not bound to the active site. The inhibitor directly competes with the substrate for binding.
Because the inhibitor in noncompetitive inhibition can bind to both the free enzyme and the enzyme-substrate complex with equal affinity, it reduces the effective concentration of functional enzyme. This effectively lowers the V<sub>max</sub>, but it doesn't alter the enzyme's affinity for the substrate.
Let's illustrate this with a hypothetical scenario:
Imagine the enzyme has two binding sites: one active site for the substrate (S) and one allosteric site for the inhibitor (I). The inhibitor binding to the allosteric site changes the conformation of the active site, making it less effective at converting the substrate into product. However, the substrate can still bind to the altered active site. The affinity (K<sub>m</sub>) remains the same, it’s just the ability to turn the substrate into product that has been reduced.
Mathematical Representation and Lineweaver-Burk Plots
The Lineweaver-Burk plot, a double reciprocal plot of the Michaelis-Menten equation (1/v<sub>0</sub> vs. 1/[S]), provides a visual representation of enzyme kinetics and different types of inhibition.
1/v<sub>0</sub> = (K<sub>m</sub>/V<sub>max</sub>)(1/[S]) + 1/V<sub>max</sub>
In noncompetitive inhibition, the Lineweaver-Burk plot shows:
-
Increased y-intercept: The y-intercept represents 1/V<sub>max</sub>. Since V<sub>max</sub> is decreased by noncompetitive inhibition, the y-intercept shifts upwards.
-
Unchanged x-intercept: The x-intercept represents -1/K<sub>m</sub>. Since K<sub>m</sub> remains unchanged, the x-intercept stays the same. This visually confirms that K<sub>m</sub> is unaffected.
The parallel lines in the Lineweaver-Burk plot for noncompetitive inhibition graphically demonstrate the unchanged K<sub>m</sub> and the decreased V<sub>max</sub>.
Biological Significance and Examples of Noncompetitive Inhibition
Noncompetitive inhibition plays a significant role in various biological processes, including:
-
Enzyme regulation: Many enzymes are regulated by noncompetitive inhibitors that fine-tune their activity in response to cellular needs.
-
Drug design: Many drugs act as noncompetitive inhibitors, targeting specific enzymes involved in disease processes. For example, some antiviral drugs inhibit viral enzymes, thereby preventing viral replication.
-
Metabolic control: Noncompetitive inhibitors can help regulate metabolic pathways by modulating the activity of key enzymes.
Specific examples of noncompetitive inhibition are less common to find explicitly described as such, since it is less common in the cell than other mechanisms. However, allosteric regulation, a form of noncompetitive inhibition where the regulatory molecule is a metabolite within the pathway, occurs frequently. Many enzymes are regulated by feedback inhibition, a process in which the end product of a metabolic pathway inhibits an earlier enzyme in the pathway.
Differentiating Noncompetitive from Other Inhibition Types
It's crucial to be able to differentiate noncompetitive inhibition from other types of reversible inhibition:
| Feature | Competitive Inhibition | Noncompetitive Inhibition | Uncompetitive Inhibition | Mixed Inhibition |
|---|---|---|---|---|
| K<sub>m</sub> | Increased | Unchanged | Decreased | Increased or Decreased |
| V<sub>max</sub> | Unchanged | Decreased | Decreased | Decreased |
| Lineweaver-Burk | Lines intersect on y-axis | Lines are parallel | Lines intersect on 2nd quadrant | Lines intersect left of y-axis |
| Inhibitor Binding | Active site | Allosteric site | Enzyme-substrate complex | Both free enzyme and complex |
Conclusion: The Invariance of K<sub>m</sub> in Noncompetitive Inhibition
Noncompetitive inhibition uniquely affects enzyme kinetics by decreasing V<sub>max</sub> without changing K<sub>m</sub>. This characteristic stems from the inhibitor's ability to bind to both the free enzyme and the enzyme-substrate complex, inducing conformational changes that reduce catalytic efficiency but don't affect the substrate's binding affinity. Understanding this mechanism is vital for interpreting enzyme kinetics data and for designing effective therapeutic interventions targeting enzyme activity. The unchanged K<sub>m</sub> in noncompetitive inhibition highlights the distinct difference between this mechanism and other types of enzyme inhibition. The Lineweaver-Burk plot effectively visualizes this unique characteristic, providing a powerful tool for analyzing and understanding enzyme inhibition patterns in various biochemical systems.
Latest Posts
Latest Posts
-
Express The Interval As An Inequality
Apr 02, 2025
-
Toward Or At The Body Surface
Apr 02, 2025
-
Thesis Statement For Narrative Essay Example
Apr 02, 2025
-
Inborn Nonspecific Defenses Include And Barriers
Apr 02, 2025
-
Jewish Murals From The First Century Ce Depict
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Why Does Noncompetitive Inhibition Not Affect Km . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
