How Many Valence Electrons Does Alkali Metals Have
Muz Play
Mar 29, 2025 · 6 min read
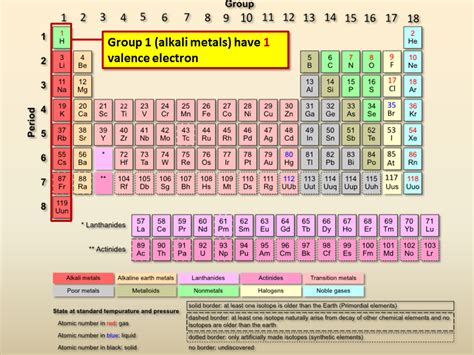
Table of Contents
How Many Valence Electrons Do Alkali Metals Have? A Deep Dive into Group 1 Elements
Alkali metals, the vibrant stars of Group 1 on the periodic table, are known for their reactivity and distinctive properties. Understanding their behavior hinges on a fundamental aspect of their atomic structure: valence electrons. This article delves deep into the world of alkali metals, explaining precisely how many valence electrons they possess and exploring the implications of this characteristic on their chemical and physical properties.
Understanding Valence Electrons: The Key to Reactivity
Before focusing specifically on alkali metals, let's establish a clear understanding of valence electrons. These are the electrons located in the outermost shell (or energy level) of an atom. They are the electrons most involved in chemical bonding, determining an element's reactivity and the types of compounds it can form. The number of valence electrons directly influences an element's position on the periodic table and its behavior within chemical reactions.
Atoms strive for stability, often achieved by attaining a full outermost electron shell, resembling the electron configuration of noble gases. This principle, known as the octet rule, dictates that atoms tend to gain, lose, or share electrons to achieve eight electrons in their valence shell (exceptions exist, especially for elements in the early periods).
Alkali Metals: A Family Portrait with One Valence Electron
The alkali metals – lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr) – are characterized by having only one valence electron. This single electron in their outermost s-orbital is loosely held and readily participates in chemical reactions. This explains their high reactivity and characteristic properties.
Electron Configuration: The Foundation of Alkali Metal Behavior
The electron configuration provides a precise picture of electron arrangement within an atom. The alkali metals' electron configurations consistently display one electron in their outermost s-orbital. For example:
- Lithium (Li): 1s²2s¹
- Sodium (Na): 1s²2s²2p⁶3s¹
- Potassium (K): 1s²2s²2p⁶3s²3p⁶4s¹
- Rubidium (Rb): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹
- Cesium (Cs): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s¹
- Francium (Fr): 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p⁶7s¹
Notice the consistent pattern: a single electron occupying the outermost s-orbital. This lone electron is easily lost, leading to the formation of a +1 ion. This is the defining characteristic of alkali metal reactivity.
The Consequences of One Valence Electron: Exploring Alkali Metal Properties
The presence of just one valence electron profoundly impacts the properties of alkali metals. Let's examine some key characteristics:
1. High Reactivity: Eager Electron Donors
The single valence electron is loosely held, making it easily lost. This tendency to lose an electron and form a +1 cation (positive ion) is the root of their high reactivity. Alkali metals readily react with water, oxygen, and halogens, often explosively. This reactivity increases as you move down the group (from lithium to francium), reflecting the decreasing ionization energy. The larger the atom, the further the valence electron is from the nucleus, and the easier it is to remove.
2. Low Ionization Energy: The Ease of Electron Loss
Ionization energy is the energy required to remove an electron from an atom. Alkali metals have exceptionally low ionization energies, signifying the ease with which they lose their single valence electron. This low energy requirement contributes significantly to their high reactivity.
3. Low Electronegativity: Unwillingness to Gain Electrons
Electronegativity measures an atom's tendency to attract electrons towards itself in a chemical bond. Alkali metals possess low electronegativities, meaning they are unlikely to gain an electron to achieve a stable octet. Instead, they prefer to lose their single electron to become positively charged ions.
4. Metallic Bonding: A Sea of Electrons
Alkali metals exhibit metallic bonding. Their valence electrons are delocalized, forming a "sea" of electrons that surrounds the positively charged metal ions. This arrangement accounts for their characteristic properties like excellent electrical and thermal conductivity, malleability, and ductility. The loosely held electrons can easily move throughout the metal structure, facilitating the flow of both heat and electricity.
5. Softness and Low Density: A Consequence of Metallic Bonding
Due to the nature of metallic bonding, alkali metals are generally soft and have low densities. The weaker electrostatic forces between the delocalized electrons and the metal ions allow the metal atoms to slide past each other easily, resulting in malleability and ductility. The low density reflects the relatively large atomic size and the loosely packed structure.
6. Characteristic Flame Colors: Excitation and Emission of Light
When heated in a flame, alkali metal atoms absorb energy, causing their valence electrons to jump to higher energy levels. As these excited electrons return to their ground state, they emit energy in the form of light at specific wavelengths, producing characteristic flame colors. For instance, lithium produces a crimson flame, sodium a bright yellow flame, and potassium a lilac flame. This phenomenon is used in analytical chemistry for identifying alkali metals.
The Impact of Valence Electrons on Chemical Reactions
The single valence electron dictates the types of chemical reactions alkali metals participate in. They primarily form ionic compounds by losing their valence electron to electronegative elements like halogens (Group 17) or oxygen (Group 16).
Reactions with Halogens: Formation of Alkali Metal Halides
When alkali metals react with halogens, they readily lose their single valence electron to the halogen atom, which readily accepts an electron to achieve a stable octet. This transfer of electrons forms an ionic bond, resulting in the formation of alkali metal halides, such as sodium chloride (NaCl, common table salt), potassium iodide (KI), and lithium fluoride (LiF). These halides are crystalline solids with high melting points due to the strong electrostatic attraction between the positively charged alkali metal ions and the negatively charged halide ions.
Reactions with Water: A Violent Reaction
The reaction of alkali metals with water is highly exothermic (releases a significant amount of heat), often leading to violent explosions. The alkali metal reacts with water, losing its electron to a hydrogen ion (H⁺) from the water, producing hydrogen gas (H₂) and an alkali metal hydroxide. For example, sodium reacts with water according to the following equation:
2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g)
The heat generated often ignites the hydrogen gas, causing an explosion. The reactivity increases down the group, with francium reacting most vigorously.
Beyond the Basics: Deeper Insights into Alkali Metal Chemistry
While the single valence electron explains the fundamental properties, a more comprehensive understanding requires considering other factors:
- Atomic Radius: The size of the atom increases down the group, resulting in weaker attraction between the nucleus and the valence electron, hence increasing reactivity.
- Ionization Energy Trends: Ionization energy decreases down the group, making it increasingly easier to remove the valence electron.
- Electronegativity Trends: Electronegativity decreases down the group, indicating a reduced tendency to gain electrons.
- Electropositivity: Alkali metals are highly electropositive; they readily lose electrons and form positive ions.
Conclusion: The Significance of a Single Electron
The presence of a single valence electron is the cornerstone of understanding the unique characteristics of alkali metals. This seemingly simple feature drives their high reactivity, low ionization energies, low electronegativities, metallic bonding, and characteristic physical and chemical properties. Studying alkali metals provides a clear illustration of how the electron configuration directly impacts the macroscopic properties of an element, highlighting the importance of valence electrons in chemistry. Understanding this fundamental principle forms the basis for predicting their behavior in various chemical reactions and exploring their diverse applications in various fields.
Latest Posts
Latest Posts
-
What Type Of Chemical Reaction Is Represented By The Equation
Apr 01, 2025
-
Why Does Noncompetitive Inhibition Not Affect Km
Apr 01, 2025
-
What Are The Factors Affecting The Rate Of Diffusion
Apr 01, 2025
-
What Is The Vsepr Geometry Of The Particle
Apr 01, 2025
-
How To Calculate Current Through Each Resistor
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Alkali Metals Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
