Which Reactions Of Glycolysis Consume Energy Under Standard State Conditions
Muz Play
Mar 15, 2025 · 5 min read
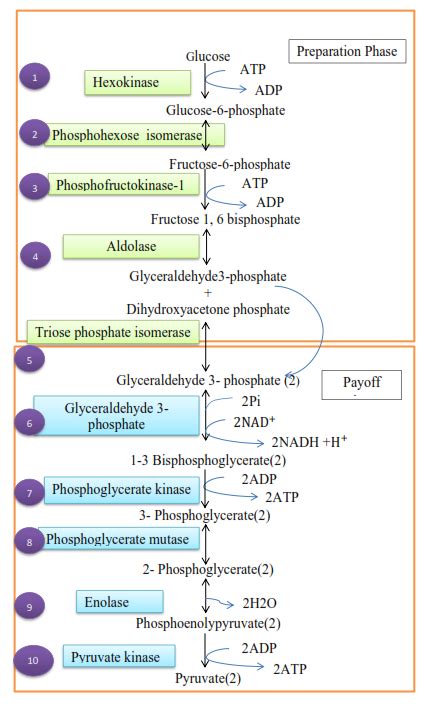
Table of Contents
Which Reactions of Glycolysis Consume Energy Under Standard State Conditions?
Glycolysis, the metabolic pathway that breaks down glucose into pyruvate, is a cornerstone of cellular energy production. While often presented as a net producer of ATP, a closer look reveals that several steps within this pathway actually consume energy under standard state conditions. Understanding these energy-requiring steps is crucial for grasping the intricate regulation and overall efficiency of glycolysis. This article will delve into the specifics of these reactions, exploring their thermodynamics and biological significance.
Understanding Standard State Conditions and Free Energy Change
Before we dive into the glycolytic reactions, it's important to define what we mean by "standard state conditions" and their relevance to free energy change (ΔG). Standard state conditions are a set of defined thermodynamic parameters used for comparing reactions. For biochemical reactions, these typically include:
- Temperature: 298 K (25°C)
- Pressure: 1 atm
- Concentration: 1 M for all reactants and products (except water, which is considered 55.5 M)
- pH: 7.0
The free energy change (ΔG) under standard state conditions (ΔG°) represents the energy released or absorbed during a reaction when all components are at their standard state concentrations. A negative ΔG° indicates an exergonic reaction (energy-releasing), while a positive ΔG° indicates an endergonic reaction (energy-consuming).
The Energy-Consuming Steps of Glycolysis
Glycolysis consists of ten enzymatic reactions, and two of them require energy input in the form of ATP hydrolysis under standard state conditions. These are:
1. Glucose Phosphorylation (Step 1): Hexokinase
The first committed step in glycolysis involves the phosphorylation of glucose to glucose-6-phosphate (G6P). This reaction is catalyzed by hexokinase and utilizes ATP:
Glucose + ATP → Glucose-6-phosphate + ADP
Under standard state conditions, this reaction has a significantly positive ΔG°, meaning it's endergonic and requires energy input. The high-energy phosphate bond of ATP is broken, providing the energy needed to drive the phosphorylation of glucose. This phosphorylation is essential because:
- Trapping Glucose: It traps glucose within the cell, preventing its diffusion out. Phosphorylated glucose cannot readily cross the cell membrane.
- Activation for Subsequent Reactions: G6P is a more reactive molecule than glucose, making it a suitable substrate for subsequent steps in glycolysis.
The positive ΔG° under standard conditions doesn't necessarily mean the reaction is non-spontaneous in vivo. The actual free energy change (ΔG) in the cell is influenced by the concentrations of reactants and products. Because the cellular concentrations of glucose and ATP are generally high, while G6P and ADP are relatively low, the ΔG in the cell becomes negative, making the reaction proceed spontaneously.
2. Fructose-6-Phosphate Phosphorylation (Step 5): Phosphofructokinase-1 (PFK-1)
The second energy-consuming step occurs during the phosphorylation of fructose-6-phosphate (F6P) to fructose-1,6-bisphosphate (F1,6BP). This reaction is catalyzed by phosphofructokinase-1 (PFK-1) and also requires ATP:
Fructose-6-phosphate + ATP → Fructose-1,6-bisphosphate + ADP
Similar to the hexokinase reaction, this step also has a positive ΔG° under standard state conditions, indicating that it's endergonic and requires energy input from ATP hydrolysis. This phosphorylation is critical because:
- Commitment to Glycolysis: This step is considered the major regulatory point in glycolysis. The large positive ΔG° of this step ensures that once F6P is phosphorylated, it is committed to completing glycolysis. The reaction is essentially irreversible under physiological conditions.
- Cleavage into Triose Phosphates: The formation of F1,6BP is essential for the subsequent cleavage of the six-carbon sugar into two three-carbon molecules (glyceraldehyde-3-phosphate and dihydroxyacetone phosphate), which then proceed through the latter half of glycolysis.
Just as with the hexokinase reaction, the actual ΔG within the cell is negative due to the cellular concentrations of reactants and products. The high ATP concentration and low F1,6BP concentration in the cell drives this reaction forward.
Why These Reactions Require Energy Input
The positive ΔG° values for these two steps under standard conditions highlight the thermodynamic challenge of phosphorylating relatively stable sugar molecules. The inherent stability of glucose and fructose-6-phosphate demands energy input to overcome the activation energy barrier for phosphorylation. The hydrolysis of ATP provides the necessary energy to accomplish this. The cellular environment, with its carefully regulated metabolite concentrations, allows these endergonic reactions to proceed spontaneously.
The Importance of Coupled Reactions in Glycolysis
The energy-consuming steps in glycolysis are tightly coupled with the subsequent energy-yielding reactions. The energy released from the later steps (primarily through substrate-level phosphorylation) more than compensates for the energy investment made in the initial two steps, resulting in a net gain of ATP.
The energy derived from the oxidation of glyceraldehyde-3-phosphate (Step 6) and the subsequent reactions are far more exergonic, driving the overall pathway forward, even though two ATP molecules were invested initially. This coupling of exergonic and endergonic reactions is a common theme in metabolism, enabling cells to perform energy-demanding tasks.
Regulation of Energy-Consuming Steps
The energy-consuming steps in glycolysis, particularly the PFK-1 reaction, are tightly regulated to meet the cell's energy needs. Several factors influence the activity of these enzymes:
- ATP levels: High ATP levels inhibit PFK-1 and hexokinase, slowing down glycolysis.
- ADP and AMP levels: High ADP and AMP levels activate PFK-1 and hexokinase, stimulating glycolysis to produce more ATP.
- Citrate levels: Citrate, an intermediate in the citric acid cycle, inhibits PFK-1, reflecting the cell's overall energy status.
- Fructose-2,6-bisphosphate: This molecule acts as a potent activator of PFK-1, stimulating glycolysis.
This intricate regulatory network ensures that glycolysis is only activated when needed and that the cell maintains an appropriate balance of ATP and other energy metabolites.
Conclusion
While glycolysis is often viewed as a pathway for generating ATP, it's crucial to acknowledge that two steps—the hexokinase and PFK-1 reactions—require energy input under standard state conditions. These endergonic reactions are essential for glucose trapping, commitment to the pathway, and preparing the substrate for subsequent energy-yielding steps. The seemingly counterintuitive energy expenditure in these steps is perfectly justified by the overall energy gain from the subsequent reactions and the carefully orchestrated regulation of the pathway. The study of these energy-consuming steps provides invaluable insights into the remarkable efficiency and finely tuned regulation of one of life's most fundamental metabolic pathways. The coupling of endergonic and exergonic reactions, along with the regulatory mechanisms in place, ensure glycolysis can adapt to the fluctuating energy demands of the cell, showcasing the elegant interplay of thermodynamics and biological control.
Latest Posts
Latest Posts
-
Which Factor Affects Congressional Approval Ratings The Most
Mar 15, 2025
-
Fourier Transform Of A Differential Equation
Mar 15, 2025
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
-
How Many Protons Does Iodine Have
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which Reactions Of Glycolysis Consume Energy Under Standard State Conditions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
