Why Activation Energy Is Not Affected By Temperature
Muz Play
Mar 17, 2025 · 5 min read
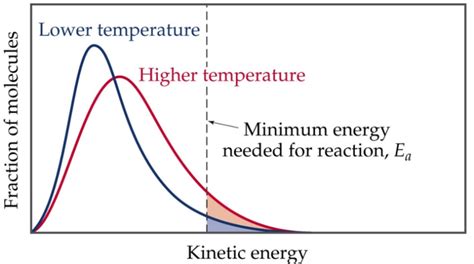
Table of Contents
Why Activation Energy Is Not Affected by Temperature
Activation energy is a fundamental concept in chemistry and physics, representing the minimum energy required for a chemical reaction to occur. Understanding its relationship with temperature is crucial for comprehending reaction rates and chemical kinetics. A common misconception is that activation energy itself changes with temperature. However, this is incorrect. Activation energy remains constant regardless of temperature changes. This article will delve deep into the reasons behind this, exploring the underlying principles and dispelling any confusion surrounding this important topic.
Understanding Activation Energy
Before we explore why activation energy isn't temperature-dependent, let's solidify our understanding of what it is. Activation energy (Ea) is the minimum amount of energy that colliding molecules must possess to initiate a chemical reaction. Think of it as the energy barrier that reactants must overcome to transform into products. This energy is needed to break existing bonds and form new ones. Molecules with kinetic energy less than Ea will simply collide and bounce apart without reacting.
The analogy of a hill: Imagine rolling a ball uphill. The height of the hill represents the activation energy. The ball needs enough energy to reach the top of the hill before it can roll down the other side. Similarly, reactant molecules require sufficient energy to overcome the activation energy barrier to form products.
The Role of Kinetic Energy
The kinetic energy of molecules is directly related to temperature. Higher temperatures mean molecules move faster and possess greater kinetic energy. This increased kinetic energy increases the probability of molecules having enough energy to surpass the activation energy barrier, thus leading to a faster reaction rate. However, this does not change the height of the energy barrier itself.
Why Temperature Doesn't Affect Activation Energy
The key to understanding why activation energy remains constant lies in its nature. Activation energy is an inherent property of the specific reaction; it's determined by the electronic structure and bonding characteristics of the reactants and the transition state. It reflects the energy required to rearrange the atoms and bonds within the molecules to form the activated complex (also known as the transition state), a high-energy intermediate state between reactants and products.
Think of it like this: The energy required to climb a mountain (activation energy) doesn't change just because the air temperature at the base of the mountain changes. The height of the mountain remains constant; only the ease with which you might climb it (the reaction rate) varies depending on your energy levels (kinetic energy of molecules).
The Arrhenius Equation and its Implications
The Arrhenius equation mathematically describes the relationship between reaction rate, temperature, and activation energy:
k = A * exp(-Ea/RT)
Where:
- k is the rate constant of the reaction
- A is the pre-exponential factor (frequency factor), representing the frequency of collisions with the correct orientation.
- Ea is the activation energy
- R is the ideal gas constant
- T is the absolute temperature
This equation clearly shows that temperature (T) affects the rate constant (k) but not the activation energy (Ea). As temperature increases, the exponential term exp(-Ea/RT) increases, leading to a higher rate constant and faster reaction rate. However, Ea remains a constant within the equation.
The Effect of Temperature on Reaction Rate, Not Activation Energy
It's crucial to distinguish between the effect of temperature on the reaction rate and its effect on activation energy. Temperature influences the reaction rate by increasing the kinetic energy of molecules, making it more likely that they possess the required activation energy to react. However, the actual activation energy barrier itself remains unchanged.
Misconceptions and Clarifications
Several misconceptions surrounding activation energy and temperature need clarification:
-
Misconception 1: Increasing temperature lowers activation energy. Clarification: This is incorrect. Increasing temperature increases the probability of molecules reaching the activation energy, but it doesn't alter the activation energy itself.
-
Misconception 2: Activation energy changes with the reaction conditions. Clarification: While reaction conditions such as presence of a catalyst can change the activation energy by providing an alternative reaction pathway with a lower energy barrier, temperature alone does not affect the intrinsic activation energy of a reaction.
-
Misconception 3: The Arrhenius equation suggests activation energy changes with temperature. Clarification: The Arrhenius equation demonstrates the relationship between temperature and reaction rate, and how temperature impacts the likelihood of a reaction occurring, but it does not suggest that Ea is variable with temperature. Ea is a constant within that equation, for a given reaction.
The Role of Catalysts
Catalysts offer a compelling illustration of how activation energy can be altered without changing the temperature. Catalysts provide an alternative reaction pathway with a lower activation energy. This means that the same reaction can proceed faster at the same temperature in the presence of a catalyst, because the energy barrier to overcome is reduced. However, this is a change in the reaction mechanism, not a temperature-induced change in the activation energy of the original reaction pathway.
Practical Implications
Understanding the constant nature of activation energy is crucial in various fields:
- Chemical Engineering: Designing reactors and optimizing reaction conditions.
- Materials Science: Studying reaction kinetics in material synthesis.
- Environmental Science: Modeling chemical reactions in the environment.
- Biology: Understanding enzyme kinetics and metabolic processes.
The constant value of activation energy allows scientists to predict reaction rates under different temperatures, making it a fundamental parameter in various scientific and engineering applications.
Conclusion
Activation energy is a fundamental property of a chemical reaction, representing the minimum energy required for it to occur. It is not affected by temperature. While temperature significantly impacts the reaction rate by influencing the kinetic energy of molecules, it does not alter the height of the activation energy barrier. The Arrhenius equation clearly illustrates this relationship, showcasing how temperature affects the rate constant without changing the activation energy. Understanding this distinction is critical for accurate modeling of reaction kinetics and various applications across diverse scientific disciplines. The misconception that activation energy is temperature-dependent stems from confusing the effect of temperature on reaction rate with its effect on the intrinsic energy barrier of the reaction. This article aims to clarify this misunderstanding and provide a comprehensive explanation of this vital concept in chemistry and physics.
Latest Posts
Latest Posts
-
Why Does Gaining An Electron Give You A Negative Charge
Mar 17, 2025
-
Converting Rectangular Coordinates To Polar Coordinates
Mar 17, 2025
-
How Much Nadh Does Glycolysis Produce
Mar 17, 2025
-
What Is A Principal Agent In Insurance
Mar 17, 2025
-
Strong And Weak Acids And Bases List
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Why Activation Energy Is Not Affected By Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
