Why Do Metals Have Low Ionization Energy
Muz Play
Apr 04, 2025 · 6 min read
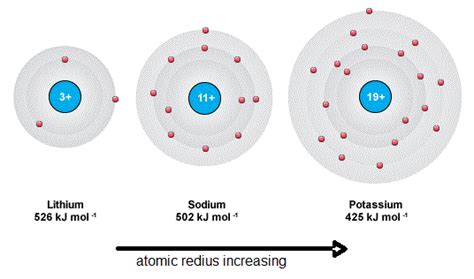
Table of Contents
Why Do Metals Have Low Ionization Energies?
Ionization energy, the energy required to remove an electron from a gaseous atom or ion, is a fundamental property that dictates an element's chemical behavior. Metals are renowned for their low ionization energies, a characteristic that underpins their many unique properties and applications. Understanding why metals exhibit this low ionization energy requires delving into the intricacies of atomic structure and electronic configuration. This article explores the factors contributing to the low ionization energies of metals, clarifying the relationship between electronic structure, metallic bonding, and the ease with which electrons are lost.
The Role of Atomic Structure and Electronic Configuration
The core reason for the low ionization energies of metals lies in their electronic configuration. Unlike non-metals, which tend to have nearly filled valence shells, metals typically possess only a few electrons in their outermost shell, also known as the valence shell. This incomplete valence shell is crucial. Electrons in the valence shell experience a relatively weak attraction to the nucleus because of the shielding effect provided by inner electrons.
Shielding Effect and Effective Nuclear Charge
The shielding effect is a phenomenon where inner electrons partially shield the outer valence electrons from the full positive charge of the nucleus. The nucleus's positive charge is partially neutralized by the negative charge of the inner electrons. This reduction in the positive charge experienced by the valence electrons is known as the effective nuclear charge.
Metals, with their multiple electron shells, exhibit a strong shielding effect. The inner electrons effectively screen the valence electrons from the strong positive pull of the nucleus. As a result, the effective nuclear charge experienced by the valence electrons is relatively low. This weaker attraction makes it easier to remove these valence electrons, leading to lower ionization energies.
Distance from the Nucleus
Another critical factor contributing to low ionization energies in metals is the distance of the valence electrons from the nucleus. In metals, the valence electrons are located further from the nucleus compared to non-metals with similar electron configurations. This greater distance reduces the electrostatic attraction between the nucleus and the valence electrons, making it easier to remove them and thus lowering ionization energy.
Metallic Bonding and Ionization Energy
The low ionization energies of metals are intimately linked to the nature of metallic bonding. Metallic bonding arises from the delocalization of valence electrons across a lattice of metal atoms. These delocalized electrons are not associated with any specific atom but are free to move throughout the metal structure, creating a "sea" of electrons. This delocalization further weakens the hold of the nucleus on the valence electrons.
Sea of Electrons and Electron Mobility
The "sea" of delocalized electrons in metals allows for high electrical and thermal conductivity. These mobile electrons easily carry charge and thermal energy throughout the structure. The ease with which these electrons move underscores the weak attraction they experience from individual atomic nuclei, reinforcing the concept of low ionization energies.
Relationship between Ionization Energy and Metallic Properties
The low ionization energies of metals are directly responsible for several characteristic metallic properties:
- Electrical Conductivity: The mobile valence electrons can easily carry an electric current when a potential difference is applied.
- Thermal Conductivity: The delocalized electrons efficiently transfer thermal energy throughout the metal structure.
- Malleability and Ductility: The non-directional nature of metallic bonds allows metal atoms to slide past each other without breaking the bonds, resulting in malleability (ability to be hammered into sheets) and ductility (ability to be drawn into wires).
- Luster: The delocalized electrons can absorb and re-emit photons of light across a wide range of wavelengths, giving metals their characteristic luster.
Comparison with Non-Metals: A Contrasting Perspective
To fully grasp the significance of low ionization energies in metals, it's beneficial to compare them with non-metals. Non-metals generally have high ionization energies because their valence shells are nearly filled. This near-completion of the valence shell means that the electrons are strongly attracted to the nucleus, making them difficult to remove.
High Ionization Energies in Non-Metals: Full Valence Shells
Non-metals tend to achieve stable electronic configurations by gaining electrons to fill their valence shells. This electron affinity, the ability to attract electrons, is high. The strong attraction between the nucleus and the electrons makes it energetically unfavorable to remove electrons, resulting in high ionization energies.
Different Bonding Mechanisms: Covalent vs. Metallic
Non-metals primarily form covalent bonds by sharing electrons, stabilizing themselves through the attainment of a stable octet configuration. This is a stark contrast to the delocalized electrons and metallic bonding characteristic of metals. The localization of electrons in covalent bonds contributes to stronger atomic interactions and higher ionization energies compared to metals.
Trends in Ionization Energy across the Periodic Table
The periodic trend in ionization energy further highlights the relationship between electronic configuration and metallic behavior. Ionization energy generally increases across a period (left to right) and decreases down a group (top to bottom) in the periodic table.
Across a Period: Increasing Nuclear Charge
As you move across a period from left to right, the nuclear charge increases, while the number of electron shells remains constant. This leads to a stronger attraction between the nucleus and the valence electrons, resulting in a higher ionization energy. Non-metals are located towards the right side of the periodic table, reflecting their higher ionization energies.
Down a Group: Increasing Shielding and Distance
As you move down a group, the nuclear charge increases, but the addition of electron shells significantly increases the shielding effect and the distance of the valence electrons from the nucleus. This outweighs the increased nuclear charge, leading to a decrease in ionization energy. This explains why elements lower down in a group, many of which are metals, tend to have lower ionization energies.
Exceptions and Complications
While the general trend of low ionization energies in metals holds true, some exceptions exist. Certain transition metals, for example, exhibit slightly higher ionization energies due to the complex interplay of electron configurations and shielding effects within their d-orbitals.
Transition Metals: D-Orbital Complications
The presence of d-orbitals in transition metals introduces complexities in electron configurations and shielding. The partially filled d-orbitals can influence the effective nuclear charge and the energy required to remove an electron, leading to deviations from the simple trends observed in main group elements.
Conclusion: A Summary of the Factors
In summary, the low ionization energies of metals are primarily attributed to the following interconnected factors:
- Few valence electrons: Metals typically have only a few electrons in their outermost shell.
- Shielding effect: Inner electrons effectively shield the valence electrons from the full positive charge of the nucleus, reducing the effective nuclear charge.
- Distance from the nucleus: Valence electrons are situated relatively far from the nucleus.
- Metallic bonding and delocalized electrons: The delocalization of valence electrons in metallic bonding further weakens the attraction between the nucleus and these electrons.
The low ionization energies of metals are fundamental to their unique physical and chemical properties, distinguishing them from non-metals and shaping their diverse applications in various technological advancements. Understanding these underlying factors provides a deeper appreciation for the behavior and importance of metals in our world.
Latest Posts
Latest Posts
-
Number Of Valence Electrons In Alkali Metals
Apr 05, 2025
-
Are Lysosomes Only In Animal Cells
Apr 05, 2025
-
Which Describes An Object In Projectile Motion
Apr 05, 2025
-
What Is The Rate Determining Step Of A Reaction
Apr 05, 2025
-
What Is The Function Of The Parental Dna In Replication
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Why Do Metals Have Low Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
