Why Does Hot Water Dissolve Things Faster
Muz Play
Mar 27, 2025 · 5 min read
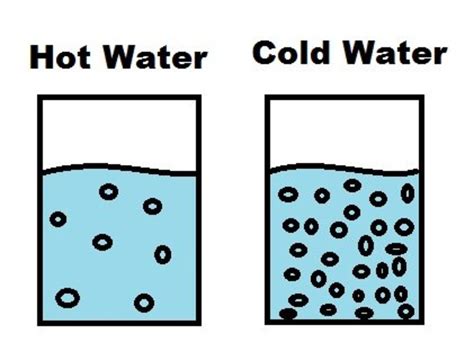
Table of Contents
Why Does Hot Water Dissolve Things Faster? A Deep Dive into the Science of Solubility
Have you ever noticed how much faster sugar dissolves in a hot cup of tea compared to a cold one? This isn't just a kitchen observation; it's a fundamental principle of chemistry that governs how substances interact and dissolve. Understanding why hot water dissolves things faster unlocks a deeper appreciation of the molecular dance that occurs at a microscopic level. This article delves into the science behind this phenomenon, exploring the key factors influencing solubility and providing real-world examples to solidify your understanding.
The Kinetic Energy Boost: Temperature's Impact on Molecular Movement
At the heart of this matter lies kinetic energy. Molecules, the tiny building blocks of matter, are constantly in motion. Temperature is essentially a measure of the average kinetic energy of these molecules. When you heat water, you're increasing the kinetic energy of its water molecules (H₂O). This increased energy translates to faster and more vigorous movement.
Increased Collision Rate: The Key to Dissolution
This heightened molecular motion is crucial for dissolution. Dissolution is the process where a solute (the substance being dissolved, like sugar or salt) breaks apart and disperses uniformly within a solvent (the substance doing the dissolving, like water). For dissolution to occur, solvent molecules must collide with solute molecules with sufficient energy to overcome the attractive forces holding the solute together.
Hot water's advantage: The increased kinetic energy in hot water means more frequent and energetic collisions between water molecules and the solute. These forceful collisions are more effective at breaking apart the solute's structure and integrating its molecules into the water.
Breaking Intermolecular Forces: The Role of Energy
The process isn't just about brute force collisions. The energy provided by heat also helps to overcome the intermolecular forces holding the solute together. These forces, such as hydrogen bonds, van der Waals forces, and dipole-dipole interactions, vary in strength depending on the solute. Hot water provides the extra energy needed to break these bonds, allowing the solute molecules to separate and disperse.
Imagine trying to separate two magnets stuck together. You'd need to apply more force than if they were only slightly attracted. Similarly, stronger intermolecular forces within the solute require more energy (provided by higher temperatures) to break apart effectively.
Beyond Temperature: Other Factors Influencing Solubility
While temperature is a dominant factor, other variables influence how quickly a substance dissolves:
1. Surface Area: More Exposure, Faster Dissolution
The surface area of the solute plays a significant role. A finely ground solute, like powdered sugar, has a much larger surface area compared to a large sugar cube. This means more solute particles are exposed to the solvent, leading to more simultaneous collisions and faster dissolution. This is why stirring a solution also helps—it constantly exposes fresh solute particles to the solvent.
2. Agitation: Encouraging Even Distribution
Stirring or agitating the solution enhances dissolution. This constant movement ensures fresh solvent molecules are constantly interacting with the solute, preventing the formation of a concentrated layer around the solute that would hinder further dissolution. Agitation effectively replenishes the solvent at the solute's surface, maintaining a high concentration gradient and facilitating a faster dissolving process.
3. Type of Solute and Solvent: Polarity Matters
The nature of the solute and solvent is crucial. Polar solvents, like water, dissolve polar solutes effectively ("like dissolves like"). Polar molecules possess an uneven distribution of charge, allowing them to interact strongly with other polar molecules. Non-polar solutes, such as fats and oils, dissolve better in non-polar solvents. The interaction between solute and solvent dictates the ease and speed of dissolution.
Real-World Examples: Observing the Effects of Hot Water
The principle of faster dissolution in hot water is evident in everyday life:
-
Making Tea or Coffee: Tea leaves or coffee grounds release their flavors and aromas much faster in hot water. The heat breaks down the cell walls of the leaves and grounds, releasing the soluble compounds into the water.
-
Dissolving Cleaning Products: Many cleaning agents, such as detergents and disinfectants, dissolve more rapidly in hot water, enabling quicker cleaning and disinfection. The increased kinetic energy helps to break down dirt and grime more efficiently.
-
Cooking: The use of hot water in cooking often speeds up the process of dissolving ingredients or extracting flavors. For instance, hot water helps dissolve salt in sauces or extract flavors from spices more quickly.
-
Pharmaceuticals: Many medications are designed to dissolve faster in hot water to enable quicker absorption by the body. This is particularly important for patients who struggle to swallow pills or tablets.
Applications in Science and Industry
The understanding of solubility and its dependence on temperature has far-reaching applications in various fields:
-
Chemical Engineering: The design of chemical processes often relies on understanding and controlling the solubility of different substances. Precise temperature control is often crucial for achieving optimal reaction rates and product yields.
-
Materials Science: The solubility of materials in different solvents at various temperatures is essential in creating new materials with desired properties. This understanding allows scientists to design processes for creating alloys, polymers, and other advanced materials.
-
Environmental Science: Understanding the solubility of pollutants in water at different temperatures helps in designing effective remediation strategies for contaminated water bodies. Temperature fluctuations can significantly impact the mobility and bioavailability of pollutants.
Conclusion: A Deeper Understanding of a Daily Phenomenon
The seemingly simple observation that hot water dissolves things faster is underpinned by a complex interplay of molecular forces and kinetic energy. Understanding this principle provides insights into various aspects of our lives, from brewing a perfect cup of tea to designing complex industrial processes. The next time you're adding sugar to your coffee, remember the microscopic dance of molecules occurring, driven by the simple power of heat. This deeper appreciation highlights the elegance and interconnectedness of scientific principles evident in our everyday experiences. Furthermore, this enhanced understanding opens up opportunities for more creative applications in various industries and expands the potential of solubility modification in our daily lives and beyond.
Latest Posts
Latest Posts
-
Journal Entry For Issuing Common Stock
Mar 30, 2025
-
Dimensional Analysis Practice Worksheet With Answers
Mar 30, 2025
-
Dissolution Of Sodium Chloride In Water
Mar 30, 2025
-
What Is A Coefficient In Chemical Equations
Mar 30, 2025
-
Do Trailing Zeros Count As Sig Figs
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Why Does Hot Water Dissolve Things Faster . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
