Dissolution Of Sodium Chloride In Water
Muz Play
Mar 30, 2025 · 5 min read
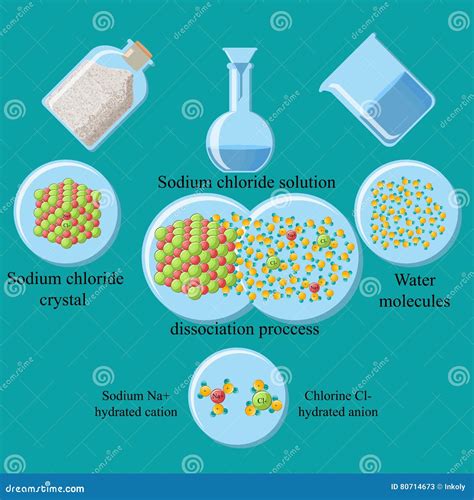
Table of Contents
The Fascinating World of Dissolving Sodium Chloride in Water: A Deep Dive
The seemingly simple act of dissolving table salt (sodium chloride, NaCl) in water is a complex process teeming with fascinating chemistry. Understanding this process is crucial not just for chemistry students, but also for anyone interested in the fundamental properties of matter and how they interact. This article will delve into the intricate details of sodium chloride dissolution, exploring the underlying forces, the steps involved, and the implications of this process in various fields.
Understanding the Players: Sodium Chloride and Water
Before diving into the dissolution process, let's examine the individual components: sodium chloride and water.
Sodium Chloride (NaCl): A Crystal Lattice of Ions
Sodium chloride, commonly known as table salt, exists as a crystalline solid. This crystal structure is characterized by a highly ordered arrangement of sodium (Na⁺) and chloride (Cl⁻) ions held together by strong electrostatic forces of attraction. These ions are held tightly in a three-dimensional lattice, giving the crystal its rigid structure and high melting point. The strong ionic bonds are the key to understanding its behavior in water.
Water (H₂O): A Polar Solvent with Unique Properties
Water is a polar solvent, meaning it possesses a partial positive charge (δ+) on the hydrogen atoms and a partial negative charge (δ-) on the oxygen atom. This polarity arises from the difference in electronegativity between oxygen and hydrogen. The bent molecular geometry further enhances this polarity, creating a dipole moment. This polarity plays a crucial role in its ability to dissolve ionic compounds like NaCl. Water molecules' ability to form hydrogen bonds with each other also contributes to its unique properties as a solvent.
The Dissolution Process: A Step-by-Step Breakdown
The dissolution of sodium chloride in water is a physical change, not a chemical change. This means that the chemical composition of NaCl and H₂O remains unchanged; only their physical state and arrangement change. The process can be broken down into several key steps:
1. Hydration of Ions: The Key to Dissolution
When NaCl crystals are added to water, water molecules begin to interact with the crystal's surface. The polar water molecules are attracted to the charged ions at the crystal surface. The partially negative oxygen atoms of water molecules are attracted to the positively charged sodium ions (Na⁺), while the partially positive hydrogen atoms are attracted to the negatively charged chloride ions (Cl⁻). This attraction is called ion-dipole interaction.
2. Overcoming Lattice Energy: Breaking Ionic Bonds
The strong electrostatic forces holding the Na⁺ and Cl⁻ ions together in the crystal lattice must be overcome for dissolution to occur. The energy required to break these bonds is known as the lattice energy. The energy released when water molecules surround and interact with the ions (hydration energy) must be greater than the lattice energy for dissolution to occur spontaneously.
3. Ion-Dipole Interactions: Stabilizing the Ions in Solution
As water molecules surround the ions, they form a hydration shell around each ion. This hydration shell stabilizes the ions in the solution, preventing them from re-associating to form the crystal lattice. The hydration process is exothermic, meaning it releases energy, contributing to the overall spontaneity of dissolution.
4. Diffusion and Homogenization: Achieving a Uniform Solution
Once the ions are separated and surrounded by water molecules, they begin to diffuse throughout the solution. This diffusion process leads to a uniform distribution of ions in the water, resulting in a homogeneous solution. The rate of diffusion depends on several factors, including temperature and the concentration gradient.
Factors Affecting the Rate of Dissolution
Several factors influence how quickly sodium chloride dissolves in water:
-
Temperature: Increasing the temperature increases the kinetic energy of both water molecules and the ions in the crystal. This leads to more frequent and energetic collisions, accelerating the dissolution process. Higher kinetic energy means water molecules can more effectively overcome the lattice energy.
-
Surface Area: A larger surface area of the NaCl crystal (e.g., using finely ground salt) exposes more ions to the water, leading to faster dissolution. More ions are available for interaction with water molecules.
-
Agitation or Stirring: Stirring the solution increases the rate of dissolution by constantly replenishing the water molecules surrounding the crystal surface. This helps to remove hydrated ions from the surface, allowing more water molecules to interact with the crystal.
-
Concentration: The rate of dissolution decreases as the concentration of NaCl in the solution increases. As more NaCl dissolves, the concentration of Na⁺ and Cl⁻ ions in the solution increases, reducing the driving force for further dissolution. This is related to the concept of saturation.
Saturation and Solubility: Understanding Limits
The amount of NaCl that can dissolve in a given amount of water at a specific temperature is limited. When the solution reaches the point where no more NaCl can dissolve, it is said to be saturated. The solubility of NaCl refers to the maximum amount of NaCl that can dissolve in a specified amount of water at a given temperature. The solubility of NaCl in water is relatively high, approximately 360 g/L at room temperature. Beyond this point, any additional NaCl added will simply settle at the bottom of the container as undissolved solid.
Applications and Implications
The dissolution of sodium chloride in water is a fundamental process with widespread applications:
-
Electrolyte Solutions: NaCl solutions are important electrolytes, conducting electricity due to the presence of freely moving ions. This property is utilized in various applications, including batteries and electrochemical processes.
-
Biological Systems: NaCl plays a vital role in biological systems, maintaining osmotic balance and influencing various physiological processes. The dissolution of NaCl in bodily fluids is crucial for cell function.
-
Industrial Processes: NaCl solutions are used extensively in various industrial processes, including food processing, chemical manufacturing, and water treatment.
-
Environmental Science: Understanding the dissolution of salts in water is critical for understanding various environmental processes, such as water salinity, soil chemistry, and the behavior of pollutants.
Conclusion: A Simple Process with Profound Implications
The seemingly simple process of dissolving sodium chloride in water is a complex interplay of forces and interactions at the molecular level. Understanding this process is fundamental to comprehending the behavior of solutions, the properties of ionic compounds, and the myriad applications of this ubiquitous compound. From the functioning of our bodies to industrial processes and environmental phenomena, the dissolution of NaCl in water remains a crucial aspect of our world. Further research continues to refine our understanding of this seemingly simple but incredibly important process.
Latest Posts
Latest Posts
-
What Happens When An Atom Gains Or Loses An Electron
Apr 01, 2025
-
How To Name Ionic And Covalent Bonds
Apr 01, 2025
-
Liquid In A Liquid Solution Example
Apr 01, 2025
-
Express The Inequality Using Interval Notation
Apr 01, 2025
-
Difference Between Hypothesis Law And Theory
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Dissolution Of Sodium Chloride In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
