Why Is Carbon A Special Element
Muz Play
Apr 05, 2025 · 7 min read
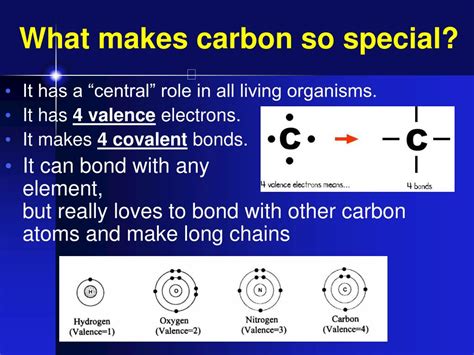
Table of Contents
Why is Carbon a Special Element? The Backbone of Life and Beyond
Carbon. It's a word we hear often, associated with everything from climate change to diamonds. But what makes this element so special, so fundamentally important to the universe and to life as we know it? It's not its abundance – oxygen and silicon are far more prevalent – but its unique properties that propel carbon to the forefront of scientific interest. This article delves deep into the remarkable characteristics of carbon, exploring its versatility, its role in organic chemistry, its diverse allotropes, and its significance in both the natural world and technological advancements.
The Unique Bonding Capabilities of Carbon
At the heart of carbon's exceptionalism lies its ability to form strong and stable bonds with itself and a wide range of other elements. This stems from its electronic configuration: it has four valence electrons, meaning it can form up to four covalent bonds. This tetravalency allows carbon atoms to link together in an almost limitless number of ways, forming chains, branches, and rings – the fundamental building blocks of organic molecules.
Covalent Bonding and its Implications
Unlike ionic bonds, which involve the transfer of electrons, covalent bonds involve the sharing of electrons between atoms. This sharing creates strong bonds that are relatively stable and resistant to breaking. The strength and stability of carbon-carbon bonds are crucial for the formation of long, complex molecules that are essential for life. The ability to form double and triple bonds further expands the possibilities of carbon's bonding, leading to a vast array of molecular structures with diverse properties.
Sp Hybridization and Molecular Geometry
The arrangement of electrons in carbon atoms, described by hybridisation, dictates the three-dimensional structure of organic molecules. Sp³, sp², and sp hybridization lead to tetrahedral, trigonal planar, and linear geometries respectively, influencing the molecule's shape, reactivity, and physical properties. This interplay between bonding and geometry is pivotal in understanding the behaviour and function of organic compounds. For example, the tetrahedral structure of methane (CH₄) is drastically different from the linear structure of carbon dioxide (CO₂), leading to contrasting physical and chemical properties.
Carbon's Role in Organic Chemistry: The Foundation of Life
Organic chemistry, the study of carbon-containing compounds, is a vast and complex field, driven by the remarkable versatility of carbon. Nearly all known living organisms are based on carbon compounds. The sheer variety of organic molecules, from simple sugars to complex proteins and nucleic acids, underscores carbon's crucial role in creating the intricate machinery of life.
The Importance of Carbon-based Macromolecules
Proteins, essential for countless biological functions, are polymers of amino acids, each containing a central carbon atom. Carbohydrates, providing energy and structural support, are composed of carbon, hydrogen, and oxygen atoms arranged in chains or rings. Lipids, including fats and oils, are crucial for energy storage and cell membranes, built around long hydrocarbon chains. And finally, nucleic acids (DNA and RNA), the carriers of genetic information, are built upon a backbone of carbon and phosphorus atoms. The diversity and complexity of these macromolecules are inextricably linked to carbon's unique bonding abilities.
Isomerism: The Power of Arrangement
The unique way carbon bonds allows for the existence of isomers, molecules with the same chemical formula but different structural arrangements. This leads to a phenomenal increase in the number of possible organic compounds, as subtle changes in arrangement can significantly alter a molecule's properties and functions. For instance, glucose and fructose have the same formula (C₆H₁₂O₆) but different structures, resulting in distinct sweetness and metabolic pathways. This isomerism is a fundamental aspect of the diversity and complexity of organic molecules and life itself.
Carbon's Allotropes: A Symphony of Forms
Carbon's ability to bond in various ways leads to the existence of different allotropes – forms of the same element with different structural arrangements. These allotropes exhibit dramatically different physical and chemical properties, showcasing the remarkable versatility of this single element.
Diamond: The Hardest Natural Substance
Diamond, renowned for its hardness and brilliance, boasts a three-dimensional network of carbon atoms bonded in a strong tetrahedral structure. Each carbon atom is linked to four others in a rigid, tightly bound lattice, resulting in the exceptional hardness and high refractive index that make diamonds highly prized. This strong, ordered structure also makes diamonds excellent heat conductors, making them valuable in industrial applications.
Graphite: The Slippery Solid
In stark contrast to diamond, graphite features a layered structure. Each layer is composed of carbon atoms arranged in a hexagonal lattice, with relatively weak forces holding the layers together. This layered structure accounts for graphite's softness and its ability to act as a lubricant. Furthermore, the delocalized electrons in graphite allow it to conduct electricity, making it a valuable component in batteries, electrodes, and pencils.
Fullerenes: The Spherical Molecules
Fullerenes, including the iconic buckminsterfullerene (C₆₀) or "buckyball," are cage-like molecules composed of carbon atoms arranged in pentagons and hexagons. Their unique structure leads to interesting properties, making them suitable for applications in materials science, medicine, and electronics. The hollow structure of fullerenes can encapsulate other atoms or molecules, opening up possibilities for drug delivery and other applications.
Carbon Nanotubes: The Ultimate Material?
Carbon nanotubes, cylindrical structures made of rolled-up graphene sheets, are exceptionally strong, lightweight, and have excellent electrical conductivity. Their unique properties make them promising materials for a wide array of applications, including advanced composites, electronics, and energy storage. Their exceptional strength-to-weight ratio and conductivity make them ideal candidates for building ultra-strong and lightweight materials for various applications.
Graphene: A Single Layer Wonder
Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, is the thinnest and strongest material known. Its exceptional electrical conductivity, high surface area, and remarkable mechanical properties have made it a revolutionary material with applications ranging from electronics to biomedical engineering. It's seen as a potential game-changer in numerous fields due to its exceptional versatility and remarkable properties.
Carbon's Importance in the Wider Universe
Carbon's significance extends far beyond Earth. It plays a vital role in the formation of stars and planets. The abundance of carbon in the cosmos allows the formation of complex organic molecules in interstellar space, suggesting that the building blocks of life might be widespread in the universe.
The Carbon Cycle and Climate Change
On Earth, carbon is a fundamental component of the carbon cycle, a biogeochemical cycle that regulates the movement of carbon between the atmosphere, oceans, land, and living organisms. Human activities, particularly the burning of fossil fuels, have significantly disrupted the carbon cycle, leading to an increase in atmospheric carbon dioxide and contributing to climate change. Understanding the carbon cycle and its interactions with the environment is crucial for addressing the challenges posed by global warming.
Carbon in Technology: Fueling Innovation
Carbon’s versatility has led to its wide use in various technologies. From the construction of buildings and bridges to the fabrication of advanced electronics, carbon-based materials are integral to modern society.
Carbon Fiber Composites: Light and Strong
Carbon fiber reinforced polymers (CFRP) are lightweight yet extremely strong composites used in aerospace, automotive, and sporting goods industries. They offer a unique combination of strength and lightness, making them ideal for applications where weight reduction is crucial, without compromising structural integrity.
Carbon Capture and Storage: Addressing Climate Change
Carbon capture and storage (CCS) technologies aim to reduce carbon dioxide emissions from power plants and industrial facilities by capturing the CO₂ and storing it underground. This approach is considered a crucial strategy for mitigating climate change by preventing the release of greenhouse gases into the atmosphere. However, it remains a developing technology with technological and economic challenges still to be addressed.
Conclusion: The Enduring Significance of Carbon
Carbon's unique properties – its tetravalency, its ability to form strong covalent bonds, and its propensity for catenation (forming long chains) – render it a truly exceptional element. This versatility is the foundation of organic chemistry, the basis of life itself, and a driving force behind countless technological advancements. From the intricate structures of biological macromolecules to the cutting-edge materials of modern technology, carbon's influence is pervasive and profound. Further research into carbon's properties and potential applications promises to unveil even more remarkable discoveries, solidifying its position as one of the most crucial elements in the universe. Its ongoing study is vital for addressing global challenges like climate change and for driving future technological innovation. The journey of understanding carbon is far from over, and its future impact on our world remains immense.
Latest Posts
Latest Posts
-
Identify The Charges Of The Protons Neutrons And Electrons
Apr 05, 2025
-
What Is A Blood Agar Plate
Apr 05, 2025
-
5 Conditions Of Hardy Weinberg Equilibrium
Apr 05, 2025
-
Is Cl More Electronegative Than C
Apr 05, 2025
-
Amino Acids With Ionizable Side Chains
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Why Is Carbon A Special Element . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
