A Pi Bond Is The Result Of The
Muz Play
Mar 24, 2025 · 7 min read
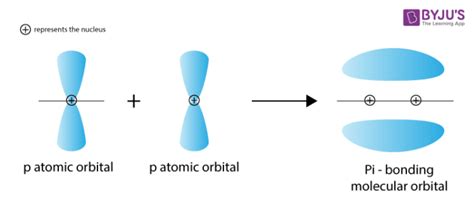
Table of Contents
A Pi Bond is the Result of: Sideways Overlap of p-Orbitals
A pi (π) bond is a type of covalent bond where two lobes of an atomic orbital on one atom overlap with two lobes of an atomic orbital on another atom. Unlike sigma (σ) bonds, which involve direct head-on overlap, pi bonds are characterized by sideways overlap of p orbitals. This sideways interaction results in a bond that is weaker and more easily broken than a sigma bond, but crucial for the structural and chemical properties of many organic and inorganic molecules. Understanding the formation and characteristics of pi bonds is fundamental to grasping the principles of organic chemistry, spectroscopy, and many areas of materials science.
The Role of p-Orbitals in Pi Bond Formation
The formation of a pi bond hinges on the unique properties of p orbitals. Unlike s orbitals, which are spherically symmetrical, p orbitals are dumbbell-shaped, possessing two lobes of opposite phase separated by a nodal plane (a region of zero electron density). It's this specific shape that allows for the sideways overlap necessary for pi bond formation.
Overlapping p-orbitals: The Key to Pi Bond Creation
When two atoms approach each other, their p orbitals can align parallel to each other. This parallel alignment allows for a sideways interaction between the lobes of the p orbitals. The crucial point here is that the electron density is concentrated above and below the internuclear axis, unlike a sigma bond where the electron density is concentrated along the internuclear axis.
Electron Density Distribution in Pi Bonds
The sideways overlap of p orbitals leads to a region of high electron density above and below the internuclear axis. This electron density is not as concentrated as in a sigma bond, resulting in a weaker bond strength. This weaker bond strength explains why pi bonds are more easily broken than sigma bonds. The presence of a nodal plane between the overlapping p orbitals also restricts the rotation around the pi bond.
Differences between Pi and Sigma Bonds
The difference between sigma and pi bonds lies primarily in the type of orbital overlap and the resulting electron density distribution.
| Feature | Sigma (σ) Bond | Pi (π) Bond |
|---|---|---|
| Orbital Overlap | Head-on overlap of atomic orbitals | Sideways overlap of p orbitals |
| Electron Density | Concentrated along the internuclear axis | Concentrated above and below the internuclear axis |
| Bond Strength | Stronger | Weaker |
| Rotation | Free rotation (generally) | Restricted rotation |
| Bond Order | Contributes 1 to the bond order | Contributes 1 to the bond order |
Multiple Bonds: A Combination of Sigma and Pi Bonds
Many molecules contain multiple bonds, such as double and triple bonds. These multiple bonds are composed of a combination of sigma and pi bonds.
Double Bonds: One Sigma, One Pi
A double bond comprises one sigma (σ) bond and one pi (π) bond. The sigma bond is formed by head-on overlap of atomic orbitals (often sp<sup>2</sup> hybridized orbitals), while the pi bond is formed by the sideways overlap of remaining unhybridized p orbitals. For example, in ethylene (C<sub>2</sub>H<sub>4</sub>), the carbon-carbon double bond consists of one sigma bond and one pi bond.
Triple Bonds: One Sigma, Two Pi
A triple bond consists of one sigma (σ) bond and two pi (π) bonds. The sigma bond is formed by the head-on overlap of hybridized orbitals (often sp hybridized orbitals), and the two pi bonds are formed by the sideways overlap of the remaining two unhybridized p orbitals. Acetylene (C<sub>2</sub>H<sub>2</sub>) provides a classic example of a molecule with a triple bond.
Delocalized Pi Bonds: Resonance and Aromaticity
In some molecules, pi electrons are not confined to a single bond but are delocalized across multiple atoms. This delocalization leads to enhanced stability and unique properties.
Resonance: Electron Delocalization
Resonance occurs when a molecule can be represented by multiple Lewis structures that differ only in the placement of electrons. These structures are resonance contributors, and the actual molecule is a hybrid of these structures. The delocalization of pi electrons across multiple atoms contributes significantly to the stability of resonance-stabilized molecules. Benzene (C<sub>6</sub>H<sub>6</sub>) is a prime example, exhibiting resonance stabilization through the delocalization of its six pi electrons.
Aromaticity: Special Stability
Aromaticity is a special type of resonance stabilization found in cyclic, planar molecules with a specific number of delocalized pi electrons (4n+2, where n is an integer – Hückel's rule). Aromatic compounds, such as benzene, are significantly more stable than expected based on their structure alone due to this delocalized pi electron system. This enhanced stability impacts their reactivity, making them less prone to addition reactions compared to their non-aromatic counterparts.
The Significance of Pi Bonds in Organic Chemistry
Pi bonds are essential for understanding many organic reactions and the properties of organic molecules.
Addition Reactions: Pi Bonds as Reactive Centers
Pi bonds are relatively weaker than sigma bonds, making them more susceptible to electrophilic and nucleophilic attack. Addition reactions involve the breaking of a pi bond and the formation of two new sigma bonds. Examples include the addition of halogens (e.g., Br<sub>2</sub>) to alkenes, a reaction where the pi bond is broken, and the halogen atoms are added across the double bond.
Polymerization: Formation of Long Chains
Pi bonds play a critical role in polymerization, the process of forming long chains of repeating units (monomers). Alkenes, with their reactive pi bonds, readily undergo addition polymerization to form polymers like polyethylene and polypropylene, materials extensively used in various applications.
Pi Bonds in Inorganic Chemistry and Materials Science
The significance of pi bonds extends beyond organic chemistry. They play a crucial role in the bonding and properties of various inorganic compounds and materials.
Metal-Ligand Bonding: Coordination Complexes
Many transition metal complexes utilize pi bonds in metal-ligand bonding. Pi backbonding, a phenomenon where electrons from metal d orbitals donate to empty ligand orbitals, stabilizes the complex and influences its reactivity.
Extended Pi Systems: Conjugated Polymers and Graphene
Extended pi systems in conjugated polymers and graphene lead to unique electronic and optical properties. These materials are often semiconductors or conductors and have applications in electronics and optoelectronics. The delocalized pi electrons in these extended systems allow for electron mobility and contribute to their conductivity.
Spectroscopic Techniques and Pi Bonds
Various spectroscopic techniques can be used to detect and characterize pi bonds.
UV-Vis Spectroscopy: Pi to Pi* Transitions
Ultraviolet-visible (UV-Vis) spectroscopy is useful for detecting pi bonds due to the ability of pi electrons to absorb UV-Vis light. The absorption corresponds to a pi to pi* transition (excitation of an electron from a bonding pi orbital to an antibonding pi* orbital). The wavelength of maximum absorption (λ<sub>max</sub>) can provide information about the extent of conjugation in a molecule. Extended conjugation shifts the absorption to longer wavelengths.
Infrared (IR) Spectroscopy: Vibrational Modes
Infrared (IR) spectroscopy can detect characteristic vibrational modes associated with pi bonds. For example, the C=C stretching frequency in alkenes usually appears around 1650 cm<sup>-1</sup>.
Conclusion
Pi bonds, arising from the sideways overlap of p orbitals, are fundamental to the structure and reactivity of a vast array of molecules. Their formation, properties, and influence on molecular behavior are crucial concepts in chemistry, influencing everything from the stability of aromatic compounds to the function of complex metal catalysts and the properties of advanced materials. The understanding of pi bonds remains an essential aspect of numerous scientific disciplines and continues to be a fertile area of research. Further research into pi bond interactions promises to lead to advancements in materials science, drug design, and other areas of technological development.
Latest Posts
Latest Posts
-
What Are The Building Blocks Of Macromolecules
Mar 25, 2025
-
Chemistry The Molecular Nature Of Matter And Change 8th Edition
Mar 25, 2025
-
Which Event Always Involves A Chemical Change
Mar 25, 2025
-
What Happens When An Atom Gains An Electron
Mar 25, 2025
-
How Does Temperature Affect Rate Of Diffusion
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about A Pi Bond Is The Result Of The . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
