A Starting Substance In A Chemical Reaction
Muz Play
Mar 23, 2025 · 6 min read
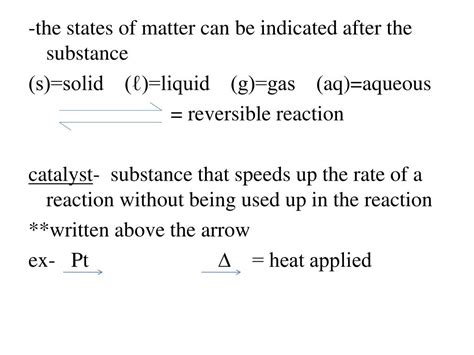
Table of Contents
Reactants: The Starting Point of Chemical Alchemy
In the captivating world of chemistry, where atoms dance and molecules waltz, understanding the fundamental building blocks is paramount. At the heart of every chemical reaction lies the reactant, the starting substance that undergoes transformation to yield products. This comprehensive exploration delves into the intricate nature of reactants, their classification, roles, and significance in various chemical processes.
Defining Reactants: The Genesis of Change
A reactant, in its simplest definition, is a substance that participates in a chemical reaction and undergoes a chemical change. It's the ingredient that gets consumed during the reaction, its atoms rearranging to form new substances. Without reactants, chemical reactions simply wouldn't happen. Think of it as the fuel that ignites the chemical fire. Reactants can be elements, compounds, or ions, and their interactions dictate the outcome of the reaction.
Differentiating Reactants from Products
It's crucial to distinguish reactants from products. While reactants are the initial substances, products are the new substances formed as a result of the chemical reaction. They represent the outcome of the rearrangement of atoms within the reactants. A simple analogy would be baking a cake: the flour, sugar, eggs, and butter are the reactants, while the finished cake is the product.
Types of Reactants: A Diverse Cast of Characters
Reactants exhibit a remarkable diversity, showcasing a wide range of properties and behaviors. Their classification can be approached from several perspectives:
1. Based on Chemical Nature:
- Elements: These are pure substances composed of only one type of atom (e.g., hydrogen, oxygen, iron). Reactions involving elements often lead to the formation of compounds.
- Compounds: These are substances composed of two or more elements chemically bonded together (e.g., water, carbon dioxide, sodium chloride). Reactions involving compounds can lead to the decomposition of the compound, or the formation of new compounds.
- Ions: These are charged atoms or molecules, formed by the gain or loss of electrons. Reactions involving ions are often driven by electrostatic forces, leading to the formation of ionic compounds or other ionic species.
2. Based on Role in the Reaction:
- Limiting Reactant: This reactant is completely consumed during the reaction, thereby limiting the amount of product that can be formed. It's like the ingredient that runs out first in a recipe, determining the maximum yield.
- Excess Reactant: This reactant is present in a larger amount than is needed for complete reaction with the limiting reactant. Some of this reactant will remain unreacted after the reaction is complete.
- Catalyst: While not technically a reactant as it isn't consumed during the reaction, a catalyst significantly accelerates the reaction rate by lowering the activation energy. It facilitates the transformation of reactants into products without being permanently altered itself.
Factors Influencing Reactant Behavior: The Chemistry of Change
Several factors significantly influence how reactants behave and interact within a chemical reaction:
1. Concentration:
The concentration of reactants directly impacts the reaction rate. Higher concentrations generally lead to more frequent collisions between reactant molecules, increasing the likelihood of successful reactions. This is governed by the law of mass action.
2. Temperature:
Temperature profoundly affects the kinetic energy of reactant molecules. Higher temperatures translate to faster molecular motion and more energetic collisions, increasing the reaction rate. However, extremely high temperatures can sometimes lead to undesirable side reactions.
3. Pressure (for gases):
For gaseous reactants, pressure plays a critical role. Increased pressure forces the gas molecules closer together, leading to more frequent collisions and a faster reaction rate.
4. Surface Area (for solids):
For solid reactants, the surface area exposed to other reactants matters. A larger surface area provides more contact points for reactions to occur, speeding up the process. This is why powdered reactants often react faster than solid chunks.
5. Presence of a Catalyst:
As mentioned earlier, catalysts dramatically accelerate reaction rates without being consumed themselves. They provide an alternative reaction pathway with a lower activation energy, thereby facilitating the transformation of reactants into products.
Reactants in Different Reaction Types: A Diverse Palette
The role and behavior of reactants vary considerably depending on the type of chemical reaction. Here are some examples:
1. Synthesis Reactions (Combination Reactions):
In these reactions, two or more reactants combine to form a single product. For example, the synthesis of water from hydrogen and oxygen:
2H₂ + O₂ → 2H₂O
Here, hydrogen and oxygen are the reactants, and water is the product.
2. Decomposition Reactions:
These reactions involve a single reactant breaking down into two or more simpler products. The decomposition of calcium carbonate is a classic example:
CaCO₃ → CaO + CO₂
In this case, calcium carbonate is the reactant, and calcium oxide and carbon dioxide are the products.
3. Single Displacement Reactions:
These reactions involve one element replacing another in a compound. For example:
Zn + 2HCl → ZnCl₂ + H₂
Zinc (Zn) replaces hydrogen (H) in hydrochloric acid (HCl). Zinc and hydrochloric acid are the reactants, and zinc chloride and hydrogen gas are the products.
4. Double Displacement Reactions (Metathesis Reactions):
These reactions involve the exchange of ions between two compounds. An example is the reaction between silver nitrate and sodium chloride:
AgNO₃ + NaCl → AgCl + NaNO₃
Silver nitrate and sodium chloride are the reactants, while silver chloride and sodium nitrate are the products.
5. Combustion Reactions:
These are exothermic reactions involving a substance reacting rapidly with oxygen, usually producing heat and light. The combustion of methane is an example:
CH₄ + 2O₂ → CO₂ + 2H₂O
Methane and oxygen are the reactants, and carbon dioxide and water are the products.
6. Acid-Base Reactions (Neutralization Reactions):
These reactions involve an acid and a base reacting to form water and a salt. A common example:
HCl + NaOH → NaCl + H₂O
Hydrochloric acid and sodium hydroxide are the reactants, while sodium chloride and water are the products.
The Significance of Reactants: The Cornerstone of Chemical Processes
Understanding reactants is not merely an academic exercise; it’s fundamental to countless aspects of our lives. From the production of everyday materials like plastics and pharmaceuticals to the intricate processes occurring in our bodies, reactants are the driving force behind it all.
Industrial Processes: Reactants are the raw materials used in countless industrial processes. The chemical industry relies heavily on precise control of reactants and reaction conditions to synthesize desired products efficiently and safely.
Biological Systems: Within living organisms, biological molecules act as reactants in a vast array of metabolic processes, enabling functions like respiration, digestion, and energy production. Enzymes act as biological catalysts, facilitating these reactions.
Environmental Chemistry: Reactants play a significant role in environmental processes, such as the formation of acid rain, ozone depletion, and pollution control. Understanding these reactions is critical for environmental remediation and protection.
Conclusion: The Ever-Evolving World of Reactants
Reactants form the very foundation of chemistry, dictating the direction and outcome of countless chemical transformations. Their diverse nature and behavior, influenced by various factors, make them a constant subject of study and exploration. As we continue to unravel the mysteries of the chemical world, a deeper understanding of reactants will undoubtedly lead to further advancements in diverse fields, from medicine and materials science to environmental protection and sustainable technologies. The study of reactants is not just about understanding chemical reactions; it’s about understanding the very essence of change and transformation in the world around us.
Latest Posts
Latest Posts
-
What Is A Cluster In Math
Mar 25, 2025
-
Gibbs Free Energy And Cell Potential
Mar 25, 2025
-
Which Of The Following Is Polar Molecule
Mar 25, 2025
-
What Is The Electron Configuration For Co
Mar 25, 2025
-
How To Identify Acid Base Reaction
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about A Starting Substance In A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
