Gibbs Free Energy And Cell Potential
Muz Play
Mar 25, 2025 · 6 min read
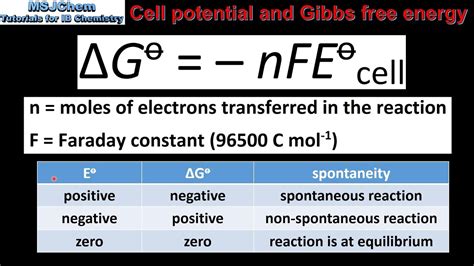
Table of Contents
Gibbs Free Energy and Cell Potential: A Deep Dive into Electrochemical Thermodynamics
Understanding the relationship between Gibbs Free Energy (ΔG) and cell potential (E) is crucial for comprehending electrochemical reactions and their spontaneity. This detailed exploration delves into the fundamental principles, connecting thermodynamic concepts with the practical applications found in batteries, fuel cells, and various electrochemical processes.
Understanding Gibbs Free Energy (ΔG)
Gibbs Free Energy, a thermodynamic potential, measures the maximum reversible work that may be performed by a system at a constant temperature and pressure. It's a powerful tool for predicting the spontaneity of a reaction. A negative ΔG indicates a spontaneous process (exergonic), meaning the reaction proceeds without external intervention. Conversely, a positive ΔG signifies a non-spontaneous process (endergonic), requiring energy input to occur. A ΔG of zero indicates a system at equilibrium.
Calculating Gibbs Free Energy
ΔG is calculated using the following equation:
ΔG = ΔH - TΔS
Where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy (heat content)
- T is the absolute temperature (in Kelvin)
- ΔS is the change in entropy (disorder)
This equation highlights the interplay between enthalpy and entropy in determining the spontaneity of a reaction. A reaction might be favored enthalpically (exothermic, ΔH < 0) but disfavored entropically (decrease in disorder, ΔS < 0). The relative magnitudes of ΔH and TΔS will ultimately dictate the sign of ΔG.
Gibbs Free Energy and Equilibrium Constant (K)
The relationship between Gibbs Free Energy and the equilibrium constant (K) is described by:
ΔG° = -RTlnK
Where:
- ΔG° is the standard Gibbs Free Energy change (at standard conditions: 298 K and 1 atm)
- R is the ideal gas constant
- T is the absolute temperature (in Kelvin)
- K is the equilibrium constant
This equation is fundamental. It shows how the standard free energy change directly relates to the equilibrium position of a reversible reaction. A large K value (K >> 1) indicates a reaction that strongly favors product formation (ΔG° << 0), while a small K value (K << 1) implies a reaction favoring reactants (ΔG° >> 0).
Cell Potential (E) and its Relationship to Gibbs Free Energy
Cell potential, also known as electromotive force (emf), is the potential difference between the two electrodes of an electrochemical cell. It's measured in volts (V) and represents the driving force for the electron flow in the external circuit. A positive cell potential indicates a spontaneous reaction, while a negative cell potential indicates a non-spontaneous reaction.
The Nernst Equation
The Nernst equation links the cell potential (E) to the standard cell potential (E°) and the reaction quotient (Q):
E = E° - (RT/nF)lnQ
Where:
- E is the cell potential
- E° is the standard cell potential
- R is the ideal gas constant
- T is the absolute temperature (in Kelvin)
- n is the number of moles of electrons transferred in the balanced redox reaction
- F is Faraday's constant (charge of one mole of electrons)
- Q is the reaction quotient
The Nernst equation is crucial because it accounts for the non-standard conditions under which electrochemical cells often operate. As the reaction proceeds, the concentrations of reactants and products change, altering the value of Q and thus the cell potential. At equilibrium (Q = K), the cell potential becomes zero (E = 0).
Connecting ΔG and E
The most important connection between Gibbs Free Energy and cell potential is given by:
ΔG = -nFE
This equation directly links the thermodynamic spontaneity (ΔG) to the electrochemical driving force (E). A negative ΔG corresponds to a positive E, signifying a spontaneous reaction. Conversely, a positive ΔG corresponds to a negative E, representing a non-spontaneous reaction. This equation is vital for predicting the feasibility of electrochemical reactions.
Standard Cell Potential (E°) and Standard Gibbs Free Energy (ΔG°)
Under standard conditions (298 K, 1 atm, 1 M concentrations), the relationship simplifies to:
ΔG° = -nFE°
The standard cell potential (E°) can be calculated from the standard reduction potentials (E°red) of the half-reactions involved:
E°cell = E°red(cathode) - E°red(anode)
This equation allows us to predict the spontaneity of a redox reaction under standard conditions by simply looking up the standard reduction potentials of the involved species in a standard reduction potential table.
Applications: Batteries and Fuel Cells
The principles of Gibbs Free Energy and cell potential have widespread applications in various electrochemical devices.
Batteries
Batteries are electrochemical cells that convert chemical energy into electrical energy. The voltage of a battery is directly related to the cell potential, determined by the specific redox reactions occurring within the battery. The battery's capacity is related to the amount of reactants available for the redox reaction, influencing the overall ΔG and the duration of operation. The spontaneity of the redox reaction, governed by ΔG and E, dictates the battery's ability to deliver electrical energy. Different battery chemistries offer varying cell potentials and energy densities, highlighting the importance of optimizing ΔG for specific applications.
Fuel Cells
Fuel cells are electrochemical devices that continuously convert chemical energy from a fuel (e.g., hydrogen) and an oxidant (e.g., oxygen) into electrical energy. The efficiency of a fuel cell is directly related to the Gibbs Free Energy change of the overall reaction. A higher cell potential implies a greater efficiency in energy conversion. The reactions in fuel cells are designed to have highly negative ΔG values, maximizing energy output. Fuel cells represent a clean and efficient alternative to traditional combustion engines, relying heavily on the principles of electrochemical thermodynamics to achieve high energy conversion efficiencies.
Beyond the Basics: Factors Influencing Cell Potential and Gibbs Free Energy
Several factors influence the cell potential and consequently the Gibbs Free Energy:
-
Temperature: As temperature changes, the equilibrium constant (K) and the reaction quotient (Q) change, affecting the cell potential (Nernst equation). This change in turn influences ΔG.
-
Concentration: Changes in reactant and product concentrations directly influence the reaction quotient (Q), which significantly alters the cell potential via the Nernst equation and subsequently ΔG.
-
Pressure: Pressure changes can affect the equilibrium constant, especially in gas-phase reactions, influencing the cell potential and ultimately ΔG.
-
pH: In many electrochemical reactions, pH plays a vital role, affecting the concentrations of involved species and, consequently, the reaction quotient (Q), cell potential, and ΔG.
Conclusion: A Powerful Interplay
The relationship between Gibbs Free Energy and cell potential is a cornerstone of electrochemistry. This connection allows for the prediction of the spontaneity of redox reactions, the calculation of cell potentials under various conditions, and the design and optimization of electrochemical devices like batteries and fuel cells. Understanding the interplay between these thermodynamic and electrochemical concepts is essential for advancements in energy storage, energy conversion, and various other applications across diverse scientific and engineering disciplines. The detailed understanding discussed in this article provides a robust foundation for further exploration into the complexities and vast applications of electrochemical thermodynamics. This in-depth analysis underscores the critical role of Gibbs Free Energy and cell potential in driving advancements across various scientific and technological fields.
Latest Posts
Latest Posts
-
What Are The Building Blocks For Fats
Mar 28, 2025
-
The Si Unit Of Energy Is The
Mar 28, 2025
-
Water Molecules Move Across Cells By
Mar 28, 2025
-
Is Ammonium Hydroxide A Strong Base
Mar 28, 2025
-
What Is The Activity Series In Chemistry
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Gibbs Free Energy And Cell Potential . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
