What Is The Activity Series In Chemistry
Muz Play
Mar 28, 2025 · 6 min read
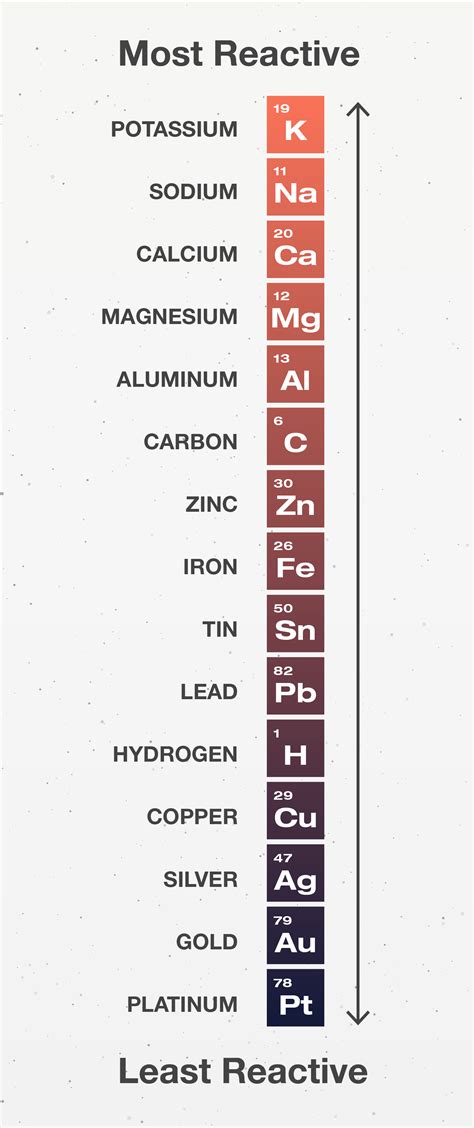
Table of Contents
- What Is The Activity Series In Chemistry
- Table of Contents
- What is the Activity Series in Chemistry? A Comprehensive Guide
- Understanding the Basics: What is the Activity Series?
- The Activity Series: A Detailed Look
- Understanding the Implications: Predicting Reactions
- Factors Affecting the Activity Series
- Applications of the Activity Series
- Extending the Activity Series: Beyond Metals
- The Activity Series and Electrochemical Series: A Comparison
- Limitations of the Activity Series
- Conclusion: Mastering the Activity Series for Chemical Success
- Latest Posts
- Latest Posts
- Related Post
What is the Activity Series in Chemistry? A Comprehensive Guide
The activity series, also known as the reactivity series, is a crucial concept in chemistry that helps us understand and predict the outcome of various chemical reactions, particularly those involving single displacement reactions. This comprehensive guide will delve deep into the activity series, exploring its principles, applications, and implications for various chemical processes.
Understanding the Basics: What is the Activity Series?
The activity series is a list of metals (and sometimes nonmetals) arranged in order of their decreasing reactivity. Reactivity, in this context, refers to the tendency of an element to lose electrons and undergo oxidation. Elements higher on the series are more reactive, meaning they readily lose electrons and participate in chemical reactions more easily than those lower on the list.
This series is essential because it allows us to predict whether a single displacement reaction will occur. A single displacement reaction, also known as a single replacement reaction, involves one element replacing another in a compound. The general form of such a reaction is: A + BC → AC + B
For a single displacement reaction to occur, the element A must be more reactive than the element B. If A is less reactive than B, the reaction will not proceed. The activity series provides the information needed to make this determination.
The Activity Series: A Detailed Look
The activity series is not a universally fixed table; slight variations may exist depending on the specific conditions (temperature, concentration, etc.). However, a common and widely accepted version includes the following metals, arranged from most to least reactive:
Highly Reactive:
- Lithium (Li): Extremely reactive alkali metal.
- Potassium (K): Highly reactive alkali metal.
- Sodium (Na): Reactive alkali metal.
- Calcium (Ca): Reactive alkaline earth metal.
- Magnesium (Mg): Moderately reactive alkaline earth metal.
- Aluminum (Al): Moderately reactive metal.
- Zinc (Zn): Moderately reactive metal.
- Iron (Fe): Relatively less reactive metal.
- Nickel (Ni): Less reactive metal.
- Tin (Sn): Relatively unreactive metal.
- Lead (Pb): Relatively unreactive metal.
- Hydrogen (H): While not a metal, hydrogen is included as a reference point for comparing the reactivity of metals with acids.
- Copper (Cu): Relatively unreactive metal.
- Silver (Ag): Unreactive metal.
- Gold (Au): Extremely unreactive metal.
- Platinum (Pt): Extremely unreactive metal.
Less Reactive (Noble Metals):
This list is not exhaustive, but it includes many commonly encountered metals. The exact placement of some elements might differ slightly depending on the source, but the overall trend remains consistent.
Understanding the Implications: Predicting Reactions
The activity series allows us to predict the outcome of various chemical reactions. Let’s consider a few examples:
Example 1: Reaction of Zinc with Hydrochloric Acid:
Zinc (Zn) is higher on the activity series than hydrogen (H). Therefore, zinc will react with hydrochloric acid (HCl) to produce zinc chloride (ZnCl₂) and hydrogen gas (H₂):
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
Example 2: Reaction of Copper with Hydrochloric Acid:
Copper (Cu) is lower on the activity series than hydrogen (H). Therefore, copper will not react with hydrochloric acid. No reaction will occur.
Example 3: Single Displacement Reaction between Metals:
Consider the reaction between iron (Fe) and copper(II) sulfate (CuSO₄). Iron is higher on the activity series than copper. This means iron will displace copper from the copper(II) sulfate solution:
Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
Example 4: A Non-Reaction:
If we reversed the previous example, placing copper with iron(II) sulfate, no reaction would occur because copper is less reactive than iron.
Cu(s) + FeSO₄(aq) → No Reaction
Factors Affecting the Activity Series
While the activity series provides a general guideline, several factors can influence the reactivity of metals:
- Temperature: Higher temperatures generally increase the rate of reaction. While it might not change the outcome of a reaction predicted by the activity series, a reaction might proceed much faster at elevated temperatures.
- Concentration: The concentration of reactants can affect the rate of reaction. Higher concentrations generally lead to faster reaction rates.
- Surface Area: A larger surface area of the reacting metal exposes more atoms to the reactants, increasing the reaction rate. Finely divided metals react faster than solid blocks of the same metal.
- Presence of Catalysts: Catalysts can speed up reactions without being consumed themselves. They can influence the rate but not necessarily alter the predicted outcome based on the activity series.
Applications of the Activity Series
The activity series has numerous practical applications in various fields:
- Corrosion Prevention: The activity series helps in selecting appropriate metals for applications where corrosion is a concern. Less reactive metals are preferred for environments where corrosion is likely.
- Extraction of Metals: The activity series guides the selection of appropriate methods for extracting metals from their ores. More reactive metals require more energy-intensive methods than less reactive ones.
- Electrochemistry: The activity series is fundamental to understanding electrochemical processes such as galvanic cells and electrolysis. The relative positions of metals in the series dictate the direction of electron flow and the potential difference in electrochemical cells.
- Predicting Reaction Outcomes: As discussed extensively, the primary application of the activity series lies in accurately predicting whether single displacement reactions will occur. This simplifies planning and executing chemical experiments.
Extending the Activity Series: Beyond Metals
While primarily focused on metals, the concept of reactivity extends to nonmetals as well. A similar series can be constructed for nonmetals based on their tendency to gain electrons (reduction). However, the reactivity series for nonmetals is less commonly used and often presented in a less structured format.
The Activity Series and Electrochemical Series: A Comparison
The activity series is closely related to, but distinct from, the electrochemical series (also known as the standard reduction potential series). The electrochemical series lists elements according to their standard reduction potentials. The standard reduction potential represents the tendency of an element to gain electrons under standard conditions. A more positive reduction potential indicates a greater tendency to gain electrons (higher reduction potential).
While both series provide information about the relative reactivity of elements, the activity series is a simpler, more qualitative representation. The electrochemical series, however, provides quantitative data (standard reduction potentials) allowing for more precise predictions of reaction spontaneity and cell potentials. The activity series can be seen as a simplified approximation of the electrochemical series.
Limitations of the Activity Series
While highly useful, the activity series has limitations:
- Oversimplification: It presents a simplified view of complex chemical interactions. It doesn't account for factors such as concentration, temperature, and catalysis in a comprehensive manner.
- Qualitative rather than quantitative: It provides a qualitative ranking of reactivity, not a quantitative measure. Therefore, it cannot accurately predict the rate of reactions or the equilibrium position.
- Limited scope: It primarily focuses on single displacement reactions and does not encompass all types of chemical reactions.
Conclusion: Mastering the Activity Series for Chemical Success
The activity series is an invaluable tool for chemists, providing a straightforward way to predict the outcome of single displacement reactions and understand the relative reactivity of elements. While it has limitations, its simplicity and utility make it a fundamental concept in chemistry education and practice. By understanding the principles outlined in this guide, you can confidently use the activity series to analyze and predict chemical reactions, fostering a deeper understanding of chemical behavior. Remembering the activity series and its applications is key to success in chemical studies and applications. Through consistent practice and further exploration, you can master this essential chemical tool and enhance your understanding of the fascinating world of chemical reactions.
Latest Posts
Latest Posts
-
Where On The Periodic Table Are The Nonmetals Located
Mar 31, 2025
-
How To Find Rate Constant From Graph
Mar 31, 2025
-
Identify The Functional Group In Each Molecule
Mar 31, 2025
-
What Signal Causes The Heart To Secrete Atrial Natriuretic Hormone
Mar 31, 2025
-
The Basic Functional Unit Of The Kidney
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Activity Series In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
