Identify The Functional Group In Each Molecule
Muz Play
Mar 31, 2025 · 8 min read
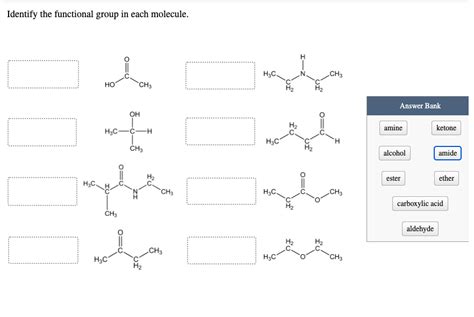
Table of Contents
Identifying Functional Groups in Molecules: A Comprehensive Guide
Organic chemistry, the study of carbon-containing compounds, hinges on understanding functional groups. These are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of that molecule. Identifying these functional groups is crucial for predicting a molecule's properties, reactivity, and potential applications. This comprehensive guide will walk you through various functional groups, their structures, and how to identify them within larger molecules.
Understanding Functional Groups: The Building Blocks of Organic Chemistry
Functional groups are like the LEGO bricks of organic chemistry. They're relatively small, specific arrangements of atoms that dictate how a molecule will behave. By learning to identify these groups, you can predict a molecule's reactivity, solubility, and other important properties without needing to understand the entire structure in minute detail. Think of it as a shortcut to understanding complex molecules.
The presence of a particular functional group determines the major chemical characteristics of a molecule. For instance, the presence of a hydroxyl group (-OH) indicates that a molecule is likely to be an alcohol, exhibiting properties like hydrogen bonding and solubility in polar solvents. Conversely, the presence of a carboxyl group (-COOH) signifies a carboxylic acid, a molecule known for its acidity.
Key Functional Groups and Their Identification
Let's delve into some of the most common functional groups, learning how to recognize them within molecular structures:
1. Hydrocarbons: The Foundation
Before exploring functional groups containing heteroatoms (atoms other than carbon and hydrogen), it's important to establish the foundation: hydrocarbons. These molecules contain only carbon and hydrogen atoms. They are further categorized into:
-
Alkanes: These are saturated hydrocarbons, meaning they contain only single bonds between carbon atoms. Their general formula is C<sub>n</sub>H<sub>2n+2</sub>. They are relatively unreactive but serve as the backbone for many other functional groups. Example: Methane (CH<sub>4</sub>), Ethane (C<sub>2</sub>H<sub>6</sub>). Identification: Look for only single bonds between carbon atoms and only carbon and hydrogen atoms.
-
Alkenes: These are unsaturated hydrocarbons containing at least one carbon-carbon double bond (C=C). Their general formula is C<sub>n</sub>H<sub>2n</sub>. The double bond introduces reactivity, making alkenes more prone to addition reactions. Example: Ethene (C<sub>2</sub>H<sub>4</sub>), Propene (C<sub>3</sub>H<sub>6</sub>). Identification: Look for at least one carbon-carbon double bond.
-
Alkynes: These are unsaturated hydrocarbons with at least one carbon-carbon triple bond (C≡C). Their general formula is C<sub>n</sub>H<sub>2n-2</sub>. Alkynes are even more reactive than alkenes. Example: Ethyne (C<sub>2</sub>H<sub>2</sub>), Propyne (C<sub>3</sub>H<sub>4</sub>). Identification: Look for at least one carbon-carbon triple bond.
-
Aromatic Hydrocarbons (Arenes): These contain a benzene ring or related structures characterized by delocalized pi electrons. They exhibit unique stability and reactivity. Example: Benzene (C<sub>6</sub>H<sub>6</sub>), Toluene (C<sub>7</sub>H<sub>8</sub>). Identification: Look for a six-membered ring with alternating single and double bonds (represented as a circle within the hexagon).
2. Oxygen-Containing Functional Groups
Oxygen is a highly electronegative atom, leading to a variety of reactive functional groups:
-
Alcohols (-OH): A hydroxyl group (-OH) bonded to a saturated carbon atom. Alcohols exhibit hydrogen bonding and are relatively polar. Example: Methanol (CH<sub>3</sub>OH), Ethanol (CH<sub>3</sub>CH<sub>2</sub>OH). Identification: Look for an -OH group attached to a carbon atom that is not part of a carbonyl group (C=O).
-
Ethers (-O-): An oxygen atom bonded to two carbon atoms. Ethers are relatively less polar than alcohols. Example: Diethyl ether (CH<sub>3</sub>CH<sub>2</sub>OCH<sub>2</sub>CH<sub>3</sub>). Identification: Look for an oxygen atom bonded to two carbon atoms.
-
Aldehydes (-CHO): A carbonyl group (C=O) bonded to at least one hydrogen atom. Aldehydes are readily oxidized. Example: Formaldehyde (HCHO), Acetaldehyde (CH<sub>3</sub>CHO). Identification: Look for a carbonyl group (C=O) where the carbon atom is bonded to at least one hydrogen atom.
-
Ketones (C=O): A carbonyl group (C=O) bonded to two carbon atoms. Ketones are less reactive than aldehydes. Example: Acetone (CH<sub>3</sub>COCH<sub>3</sub>), Propanone (CH<sub>3</sub>COCH<sub>3</sub>). Identification: Look for a carbonyl group (C=O) where the carbon atom is bonded to two other carbon atoms.
-
Carboxylic Acids (-COOH): A carboxyl group (-COOH), which is a combination of a carbonyl group and a hydroxyl group. Carboxylic acids are acidic and readily ionize. Example: Acetic acid (CH<sub>3</sub>COOH). Identification: Look for a -COOH group.
-
Esters (-COO-): Formed by the reaction between a carboxylic acid and an alcohol. Esters often have pleasant aromas. Example: Ethyl acetate (CH<sub>3</sub>COOCH<sub>2</sub>CH<sub>3</sub>). Identification: Look for a -COO- group.
3. Nitrogen-Containing Functional Groups
Nitrogen is another important heteroatom, forming several significant functional groups:
-
Amines (-NH<sub>2</sub>, -NHR, -NR<sub>2</sub>): Nitrogen atoms bonded to one, two, or three carbon atoms (primary, secondary, and tertiary amines, respectively). Amines are basic. Example: Methylamine (CH<sub>3</sub>NH<sub>2</sub>), Dimethylamine (CH<sub>3</sub>)<sub>2</sub>NH. Identification: Look for a nitrogen atom bonded to one or more carbon atoms.
-
Amides (-CONH<sub>2</sub>, -CONHR, -CONR<sub>2</sub>): A carbonyl group bonded to a nitrogen atom. Amides are less basic than amines. Example: Acetamide (CH<sub>3</sub>CONH<sub>2</sub>). Identification: Look for a -CON- group bonded to a carbon and a nitrogen.
-
Nitriles (-CN): A carbon atom triple-bonded to a nitrogen atom. Nitriles are relatively unreactive but can be hydrolyzed to carboxylic acids. Example: Acetonitrile (CH<sub>3</sub>CN). Identification: Look for a -C≡N group.
4. Other Important Functional Groups
-
Halogens (F, Cl, Br, I): Halogens bonded to carbon atoms. These increase the polarity of the molecule. Example: Chloromethane (CH<sub>3</sub>Cl). Identification: Look for F, Cl, Br, or I atoms bonded to carbon.
-
Sulfhydryls (-SH): A sulfur atom bonded to a hydrogen atom. These groups are found in thiols, which have a characteristic odor. Example: Methanethiol (CH<sub>3</sub>SH). Identification: Look for an -SH group.
-
Phosphate (-PO<sub>4</sub>): Essential in biological molecules like DNA and ATP. Identification: Look for a -PO<sub>4</sub> group.
Strategies for Identifying Functional Groups in Complex Molecules
Identifying functional groups in complex molecules requires a systematic approach. Here’s a step-by-step strategy:
-
Identify the Carbon Skeleton: First, trace the carbon backbone of the molecule. This will give you a basic framework to work with.
-
Look for Heteroatoms: Identify atoms other than carbon and hydrogen (oxygen, nitrogen, sulfur, halogens, phosphorus). These atoms are often the center of functional groups.
-
Examine the Bonding: Carefully examine the bonds surrounding the heteroatoms. Double and triple bonds are crucial for recognizing certain functional groups (C=O, C≡N, C=C).
-
Recognize Characteristic Patterns: Familiarize yourself with the characteristic patterns of common functional groups (e.g., -OH for alcohols, -COOH for carboxylic acids).
-
Break Down the Molecule: If the molecule is very large and complex, try breaking it down into smaller, more manageable sections. Identify the functional groups within each section.
-
Consider the Context: Sometimes, the context within the molecule can influence the properties of a functional group. For example, the reactivity of a hydroxyl group can change depending on its location within a larger molecule.
Practical Applications of Functional Group Identification
The ability to identify functional groups is not just an academic exercise; it has significant practical applications:
-
Drug Discovery and Development: Pharmaceutical scientists rely heavily on functional group analysis to design and synthesize new drugs. Understanding the functional groups present in a molecule is critical for predicting its biological activity and potential side effects.
-
Material Science: Functional groups play a vital role in determining the properties of materials. By modifying the functional groups in a polymer, for instance, scientists can tailor its properties for specific applications.
-
Chemical Synthesis: Organic chemists use functional group analysis to plan and execute chemical reactions. Knowing which functional groups are present in a molecule allows them to predict how it will react with other molecules.
-
Environmental Chemistry: Identifying functional groups in pollutants is crucial for developing effective remediation strategies.
Conclusion: Mastering Functional Group Identification
Mastering the identification of functional groups is a cornerstone of success in organic chemistry. By diligently studying the structures and characteristics of these key building blocks, you gain the ability to predict the properties and reactivity of a vast array of molecules. This skill is essential for anyone pursuing a career in chemistry, biology, medicine, or related fields. Remember to practice consistently, working through various examples and challenging yourself with more complex molecules to solidify your understanding. This comprehensive guide provides a strong foundation, allowing you to confidently tackle the challenges of identifying functional groups in any organic molecule you encounter.
Latest Posts
Latest Posts
-
Name The Structural And Functional Unit Of All Living Things
Apr 02, 2025
-
Where Is Oxide On The Periodic Table
Apr 02, 2025
-
How To Find The Critical Value Of R
Apr 02, 2025
-
Which Part Of Amino Acid Is Always Acidic
Apr 02, 2025
-
To Increase The Concentration Of A Solution You Could
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Identify The Functional Group In Each Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
