Is Ammonium Hydroxide A Strong Base
Muz Play
Mar 28, 2025 · 5 min read
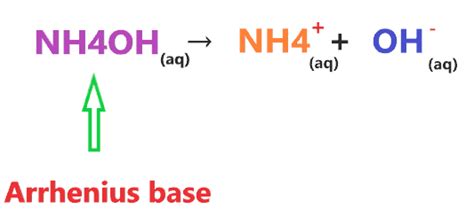
Table of Contents
Is Ammonium Hydroxide a Strong Base? Understanding Weak Bases and Their Properties
Ammonium hydroxide, also known as aqueous ammonia, is a common chemical compound often encountered in various household and industrial applications. A frequent question that arises regarding this substance is whether it's a strong base. The answer, simply put, is no. Ammonium hydroxide is considered a weak base. Understanding why requires a deeper dive into the concepts of strong and weak bases, their behavior in solution, and the specific properties of ammonium hydroxide.
Understanding Strong vs. Weak Bases
The strength of a base is determined by its ability to dissociate completely in water, releasing hydroxide ions (OH⁻).
Strong bases dissociate almost completely into their constituent ions when dissolved in water. This means a high percentage of the base molecules break apart, releasing a large number of hydroxide ions. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)₂). These bases have high pH values and readily react with acids.
Weak bases, on the other hand, only partially dissociate in water. A significant portion of the base molecules remain intact, meaning only a small number of hydroxide ions are released. This results in a lower pH compared to strong bases. The equilibrium between the undissociated base and its ions plays a crucial role in determining the base's strength.
The Behavior of Ammonium Hydroxide in Water
Ammonium hydroxide is formed when ammonia gas (NH₃) dissolves in water. The ammonia molecule reacts with water molecules according to the following equilibrium reaction:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
This reaction shows that only a small fraction of ammonia molecules react with water to form ammonium ions (NH₄⁺) and hydroxide ions (OH⁻). Most of the ammonia remains in its molecular form (NH₃). The equilibrium lies far to the left, indicating that the concentration of hydroxide ions is relatively low. This incomplete dissociation is the defining characteristic of a weak base.
Equilibrium Constant (Kb) and Weak Bases
The extent to which a weak base dissociates is quantified by its base dissociation constant (Kb). Kb is the equilibrium constant for the base dissociation reaction. A smaller Kb value indicates a weaker base, meaning it dissociates less readily. Ammonium hydroxide has a relatively small Kb value, further confirming its weak base nature.
pH and pOH of Ammonium Hydroxide Solutions
Because ammonium hydroxide is a weak base, its solutions exhibit a pH significantly less than 14 (the pH of a strong base solution). The pH of an ammonium hydroxide solution depends on its concentration. More concentrated solutions have a slightly higher pH (though still basic) than dilute solutions due to the higher concentration of hydroxide ions, but even at high concentrations, it will never reach a pH close to 14.
Factors Affecting the Strength of Ammonium Hydroxide
While the inherent nature of ammonium hydroxide classifies it as a weak base, some factors can influence its apparent strength:
-
Concentration: Higher concentrations of ammonium hydroxide will produce a slightly higher concentration of OH⁻ ions, leading to a slightly higher pH. However, even at high concentrations, it remains a weak base.
-
Temperature: Increasing the temperature can slightly increase the dissociation of ammonium hydroxide. However, this effect is relatively small and doesn't change the fundamental nature of ammonium hydroxide as a weak base.
-
Presence of other ions: The presence of other ions in the solution can influence the equilibrium of the dissociation reaction through the common ion effect. Adding ammonium salts, for example, will suppress the dissociation of ammonium hydroxide, making it appear even weaker.
Applications of Ammonium Hydroxide: Leveraging its Weak Base Properties
Despite being a weak base, ammonium hydroxide finds numerous applications, leveraging its unique properties:
-
Cleaning Products: Ammonium hydroxide's mild basic nature makes it suitable for cleaning various surfaces, including glass, countertops, and floors. Its effectiveness comes from its ability to dissolve grease and grime without being excessively harsh.
-
Fertilizers: In agriculture, ammonium hydroxide serves as a source of nitrogen, a crucial nutrient for plant growth. The weak base nature ensures it doesn't damage plant tissues.
-
Textile Industry: Ammonium hydroxide plays a role in treating textiles, often acting as a pH regulator or involved in specific dyeing processes. Its mild alkalinity makes it suitable for delicate fabrics.
-
Food Industry: In limited instances, it can be used as a food additive (though regulated strictly), often as a pH adjuster or in specific food processing techniques.
Comparing Ammonium Hydroxide to Strong Bases
The differences between ammonium hydroxide and strong bases like sodium hydroxide are significant:
| Feature | Ammonium Hydroxide (Weak Base) | Sodium Hydroxide (Strong Base) |
|---|---|---|
| Dissociation | Partial | Complete |
| Hydroxide Ions | Low concentration | High concentration |
| pH | Slightly basic (less than 14) | Close to 14 |
| Corrosiveness | Mild | Highly corrosive |
| Handling | Relatively safe (with precautions) | Requires careful handling |
Safety Precautions when Handling Ammonium Hydroxide
While ammonium hydroxide is not as corrosive as strong bases, it's still essential to handle it carefully. Always wear appropriate personal protective equipment (PPE), including gloves and eye protection. Avoid skin contact and inhalation. In case of accidental contact, immediately flush the affected area with plenty of water.
Conclusion: Ammonium Hydroxide Remains a Weak Base
In conclusion, despite its various applications, ammonium hydroxide is unequivocally a weak base. Its incomplete dissociation in water, low concentration of hydroxide ions, and relatively low pH value clearly distinguish it from strong bases. Understanding this fundamental property is crucial for safe handling, appropriate application, and effective use in various industrial and household settings. The information presented here highlights its characteristics, behaviors, and differences from strong bases, providing a comprehensive understanding of this important chemical compound. Always prioritize safety when working with any chemical, including the seemingly mild ammonium hydroxide.
Latest Posts
Latest Posts
-
What Happens To An Animal Cell In A Isotonic Solution
Mar 31, 2025
-
Atom That Has Gained Or Lost Electrons
Mar 31, 2025
-
Cuantas Onzas Tiene Un Cuarto De Galon
Mar 31, 2025
-
Work Done By A Varying Force
Mar 31, 2025
-
The Rna Components Of Ribosomes Are Synthesized In The
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Is Ammonium Hydroxide A Strong Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
