Water Molecules Move Across Cells By
Muz Play
Mar 28, 2025 · 6 min read
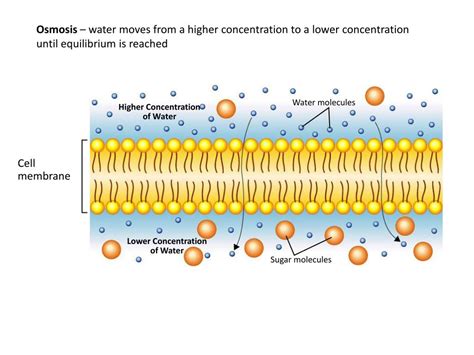
Table of Contents
Water Molecules Move Across Cells By: A Deep Dive into Osmosis and Diffusion
Water, the elixir of life, is essential for all cellular processes. But how does this vital molecule traverse the intricate membranes that encase our cells? The answer lies in two primary mechanisms: osmosis and diffusion. Understanding these processes is crucial for comprehending cellular function, physiology, and even disease. This article will delve into the intricacies of how water molecules move across cell membranes, exploring the underlying principles, influencing factors, and their implications.
Passive Transport: The Engine of Water Movement
Before we dive into the specifics of osmosis and diffusion, it's important to understand that the movement of water across cell membranes is largely a form of passive transport. This means that it doesn't require the cell to expend energy (ATP). Instead, it relies on the inherent properties of water molecules and the concentration gradients across the membrane.
Diffusion: The Random Walk of Water
Diffusion is the fundamental principle governing the movement of many substances, including water, across membranes. It's based on the random thermal motion of molecules. Water molecules, like all molecules, are constantly in motion, colliding with each other and their surroundings. This constant movement leads to a net movement of water from an area of high concentration to an area of low concentration. This movement continues until equilibrium is reached, meaning the concentration of water is equal on both sides of the membrane.
Factors Affecting Diffusion:
- Concentration Gradient: The steeper the concentration gradient (the bigger the difference in water concentration), the faster the rate of diffusion.
- Temperature: Higher temperatures increase the kinetic energy of water molecules, leading to faster diffusion.
- Membrane Permeability: The permeability of the cell membrane to water significantly impacts the rate of diffusion. A more permeable membrane allows water to pass through more easily.
- Surface Area: A larger surface area allows for more water molecules to cross the membrane simultaneously.
- Distance: The shorter the distance water needs to travel, the faster the diffusion rate.
Osmosis: Water's Special Case of Diffusion
Osmosis is a specialized type of diffusion that specifically deals with the movement of water across a selectively permeable membrane. This membrane allows water to pass through but restricts the movement of certain solutes (dissolved substances). In osmosis, water moves from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). Essentially, water moves to dilute the area with the higher solute concentration.
Osmotic Pressure:
The pressure required to prevent the net movement of water across a selectively permeable membrane is called osmotic pressure. It's a measure of the tendency of water to move into a solution due to the presence of solutes. A solution with a high solute concentration will exert a higher osmotic pressure than a solution with a low solute concentration.
Tonicity and its Impact on Cells:
The concept of tonicity describes the relative concentration of solutes in two solutions separated by a selectively permeable membrane. It has significant implications for cells:
- Isotonic Solution: The solute concentration inside and outside the cell is equal. There is no net movement of water, and the cell maintains its normal shape.
- Hypotonic Solution: The solute concentration outside the cell is lower than inside the cell. Water moves into the cell, causing it to swell and potentially lyse (burst).
- Hypertonic Solution: The solute concentration outside the cell is higher than inside the cell. Water moves out of the cell, causing it to shrink and crenate.
Aquaporins: The Water Channels
While diffusion and osmosis explain the why of water movement, the how involves specialized protein channels embedded within the cell membrane: aquaporins. These remarkable proteins form pores that selectively allow water molecules to pass through the membrane much faster than they could through simple diffusion.
Structure and Function of Aquaporins:
Aquaporins are tetrameric proteins, meaning each channel is composed of four identical subunits. Each subunit contains a pore that is highly selective for water molecules. The structure of the pore prevents the passage of ions and other solutes, ensuring that only water crosses the membrane through these channels.
Regulation of Aquaporin Expression:
The number of aquaporins expressed in a cell membrane can vary depending on the cell's needs and the physiological conditions. This regulation allows cells to fine-tune their water permeability, adapting to changes in their environment.
Importance of Aquaporins in Various Physiological Processes:
Aquaporins play crucial roles in numerous biological processes, including:
- Kidney function: Reabsorption of water from the filtrate in the kidneys.
- Brain function: Maintaining water balance in the brain.
- Plant growth: Water uptake and transport in plants.
- Tear production: Maintaining the hydration of the eyes.
- Saliva production: Maintaining oral hydration.
Beyond Osmosis and Diffusion: Other Mechanisms
While osmosis and diffusion facilitated by aquaporins are the primary mechanisms, other processes can also contribute to water movement across cell membranes:
- Bulk flow: Water can move in bulk through larger channels or openings in the cell membrane, particularly in specialized cells. This is often driven by pressure differences.
- Active transport: While less common for water movement, certain specialized proteins can actively transport water across membranes against its concentration gradient. This requires energy expenditure by the cell.
Clinical Significance: Water Imbalance and Disease
Disruptions to the delicate balance of water movement across cell membranes can have significant clinical implications. For example:
- Dehydration: A lack of water in the body leads to cellular shrinkage and impaired function.
- Water intoxication (hyponatremia): Excessive water intake can dilute electrolytes, causing cells to swell and potentially leading to neurological complications.
- Edema: Fluid buildup in tissues due to an imbalance in water movement.
- Kidney diseases: Impaired kidney function can lead to an inability to regulate water balance.
- Cystic fibrosis: Mutations in certain membrane proteins can affect water transport in various tissues.
Conclusion: A Dynamic Equilibrium
Water movement across cell membranes is a dynamic and finely regulated process, essential for maintaining cellular homeostasis and overall organismal health. Osmosis and diffusion, facilitated by aquaporins, are the primary mechanisms, but other processes can also play a role. Understanding these processes is crucial for comprehending cellular function, physiology, and the development of various diseases. The continuing research into the intricate details of water transport will undoubtedly shed more light on this fundamental aspect of life itself. Further exploration into the regulation of aquaporins, the interplay between different transport mechanisms, and the clinical consequences of water imbalance promise to yield valuable insights for advancing human health and understanding the biological world around us. Future studies might focus on developing novel therapies targeting aquaporins for treating conditions like edema or dehydration, or even creating new bioengineering applications based on the remarkable properties of these water channels. The journey of understanding water transport across cell membranes is far from over, and exciting discoveries lie ahead.
Latest Posts
Latest Posts
-
What Happens To An Animal Cell In A Isotonic Solution
Mar 31, 2025
-
Atom That Has Gained Or Lost Electrons
Mar 31, 2025
-
Cuantas Onzas Tiene Un Cuarto De Galon
Mar 31, 2025
-
Work Done By A Varying Force
Mar 31, 2025
-
The Rna Components Of Ribosomes Are Synthesized In The
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Water Molecules Move Across Cells By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
