What Is The Electron Configuration For Co
Muz Play
Mar 25, 2025 · 6 min read
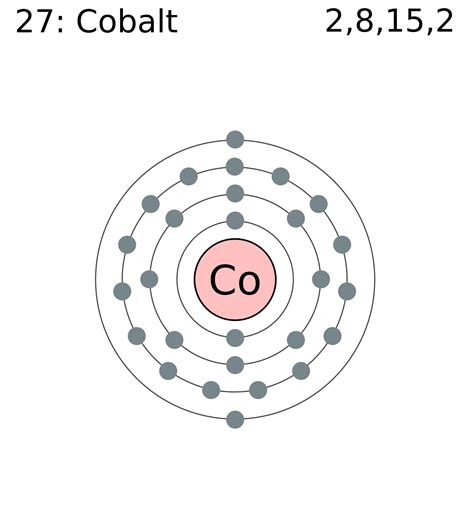
Table of Contents
What is the Electron Configuration for Co (Cobalt)? A Deep Dive into Atomic Structure
Cobalt (Co), a transition metal with atomic number 27, boasts a fascinating electron configuration that dictates its unique chemical and physical properties. Understanding this configuration is key to grasping its behavior in various contexts, from its role in biological systems to its applications in advanced materials. This article will delve into the electron configuration of cobalt, exploring its intricacies and implications.
Understanding Electron Configuration
Before diving into cobalt's specific configuration, let's establish a foundational understanding of the concept. Electron configuration describes the arrangement of electrons in an atom's electron shells and subshells. These arrangements follow specific rules dictated by quantum mechanics, including the Aufbau principle (filling orbitals from lowest to highest energy), Hund's rule (maximizing unpaired electrons in a subshell), and the Pauli exclusion principle (no two electrons can have the same four quantum numbers).
The electron configuration is typically represented using a notation that specifies the principal quantum number (n), the subshell (s, p, d, or f), and the number of electrons in each subshell. For example, 1s² indicates two electrons in the 1s subshell.
Determining the Electron Configuration of Cobalt (Co)
Cobalt, with an atomic number of 27, possesses 27 electrons. To determine its electron configuration, we systematically fill the electron shells and subshells according to the Aufbau principle.
The order of filling orbitals is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... However, there are some exceptions, especially with transition metals like cobalt.
Therefore, the complete electron configuration for Cobalt is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁷.
Let's break this down:
- 1s²: Two electrons fill the first energy level's s subshell.
- 2s²: Two electrons fill the second energy level's s subshell.
- 2p⁶: Six electrons fill the second energy level's p subshell.
- 3s²: Two electrons fill the third energy level's s subshell.
- 3p⁶: Six electrons fill the third energy level's p subshell.
- 4s²: Two electrons fill the fourth energy level's s subshell. Note: Although the 3d subshell has a slightly higher energy than the 4s subshell, the 4s subshell fills first due to its lower principal quantum number (n=4 vs. n=3) and the overall energy minimization principle.
- 3d⁷: Seven electrons partially fill the third energy level's d subshell. This is where the unique properties of cobalt begin to manifest.
The Significance of the 3d Subshell in Cobalt's Properties
The partially filled 3d subshell is the key to understanding cobalt's characteristics. This incomplete d-orbital configuration is responsible for:
1. Variable Oxidation States:
Cobalt exhibits multiple oxidation states, most commonly +2 and +3. The presence of seven electrons in the 3d subshell allows for the easy loss or gain of electrons to achieve a stable configuration, resulting in these variable oxidation states. This is a defining characteristic of transition metals. For example, in Co²⁺, two electrons are lost, typically from the 4s subshell, leaving a 3d⁷ configuration, while in Co³⁺, three electrons are lost, typically two from 4s and one from 3d, resulting in a 3d⁶ configuration. These different oxidation states lead to different chemical properties and behaviors.
2. Magnetic Properties:
The unpaired electrons in the 3d subshell of cobalt contribute to its magnetic properties. These unpaired electrons create a magnetic moment, leading to cobalt's paramagnetism. This means it is weakly attracted to a magnetic field. Some cobalt compounds and alloys exhibit stronger magnetic properties, making them crucial components in various magnetic applications.
3. Catalytic Activity:
The partially filled d-orbitals in cobalt facilitate its ability to act as a catalyst. This is because these orbitals can readily accept or donate electrons during chemical reactions, lowering the activation energy and accelerating the reaction rate. Cobalt's catalytic properties are utilized extensively in industrial processes and chemical synthesis.
4. Complex Formation:
The d-orbitals in cobalt allow it to form a variety of coordination complexes. This is a crucial aspect of its chemistry. Cobalt can form complexes with ligands (molecules or ions that bind to the central metal atom) and these complexes exhibit unique geometries, colors, and properties depending on the type and arrangement of ligands. For instance, many cobalt complexes with different ligands showcase vibrant colors due to d-orbital electron transitions, absorbing and emitting light in the visible spectrum.
5. Color:
The d-d transitions within the incomplete 3d subshell allow for the absorption of certain wavelengths of light and transmission of others. This process causes cobalt compounds to exhibit a range of characteristic colors.
Cobalt's Importance in Different Fields
The unique properties stemming from its electron configuration make cobalt an essential element across diverse fields:
-
Biochemistry: Cobalt is an essential component of vitamin B12, a vital coenzyme involved in various metabolic processes. The unique electron configuration of the cobalt ion at the center of vitamin B12 is crucial for its biological activity.
-
Metallurgy: Cobalt is used in the production of various alloys, particularly superalloys, that exhibit exceptional high-temperature strength and corrosion resistance. These alloys find applications in jet engines, gas turbines, and other high-performance components.
-
Catalysis: Cobalt-based catalysts play crucial roles in various industrial processes, including the Fischer-Tropsch synthesis of hydrocarbons from synthesis gas and hydroformylation reactions.
-
Magnetism: Cobalt is a key component in permanent magnets, exhibiting high coercivity and remanence. These magnets are used in various applications, including electric motors, sensors, and data storage devices.
-
Pigments: Cobalt compounds are used as pigments in paints, ceramics, and glass, producing various shades of blue, green, and violet.
Variations and Exceptions in Electron Configuration
While the standard electron configuration for cobalt is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁷, subtle variations can occur depending on the chemical environment. For example, in some compounds, the electron configuration might deviate slightly due to ligand field effects. Ligands can affect the energy levels of the d-orbitals, influencing electron occupancy and ultimately affecting the properties of the cobalt complex.
Conclusion
The electron configuration of cobalt, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁷, is not merely a theoretical construct; it's the blueprint for its remarkable properties. The partially filled 3d subshell is the engine driving cobalt's versatility, contributing to its variable oxidation states, magnetic behavior, catalytic activity, complex formation, and distinctive color. Understanding this configuration is crucial for appreciating cobalt's significance in various scientific and technological applications, ranging from biological systems to advanced materials. The detailed study of its electron configuration helps us unravel the mysteries of its multifaceted nature and its impact on our world.
Latest Posts
Latest Posts
-
Does Oxidation Occur At The Anode
Mar 28, 2025
-
The Membrane Holds The Coils Of The Small Intestine
Mar 28, 2025
-
Organelles In Eukaryotic Cells Answer Key
Mar 28, 2025
-
Lewis Dot Diagram For Ionic Bonding Between Li And F
Mar 28, 2025
-
What Is The Relationship Between Avogadros Number And The Mole
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Co . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
