According To Dalton's Atomic Theory An Atom Is
Muz Play
Mar 15, 2025 · 7 min read
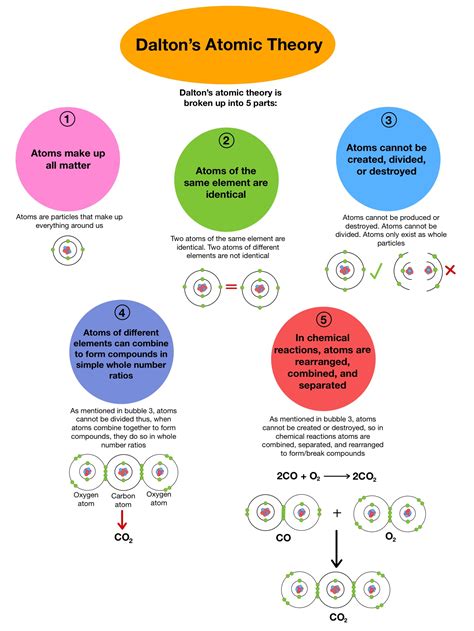
Table of Contents
According to Dalton's Atomic Theory, an Atom Is... A Deep Dive into the Foundations of Modern Chemistry
John Dalton's atomic theory, proposed in the early 1800s, revolutionized our understanding of matter. While not entirely accurate by today's standards, it laid the groundwork for modern chemistry and remains a crucial stepping stone in our scientific journey. This article will explore Dalton's atomic theory in detail, examining its postulates, its impact on the scientific community, and its limitations in light of subsequent discoveries.
Dalton's Postulates: A Foundation for Understanding Matter
Dalton's atomic theory rests on several key postulates, each contributing to a new paradigm in the understanding of matter:
1. All Matter is Made of Atoms: The Indivisible Building Blocks
Dalton's first postulate boldly declared that all matter, regardless of its form, is composed of indivisible particles called atoms. This was a radical departure from previous philosophical concepts that considered matter infinitely divisible. This assertion, though later proven to be partially incorrect, provided a fundamental framework for understanding the building blocks of the universe. It was the cornerstone upon which all subsequent postulates were built. The simplicity and power of this foundational statement propelled the acceptance of atomic theory within the scientific community.
2. Atoms of a Given Element are Identical: The Essence of an Element
Dalton proposed that all atoms of a particular element are identical in mass and properties. This implied that atoms of oxygen, for instance, were all alike in terms of mass and other inherent characteristics. This postulate emphasized the distinct nature of different elements, highlighting the unique identity of each atom type. It provided a clear distinction between the fundamental building blocks of different substances, streamlining the analysis of chemical reactions.
3. Atoms of Different Elements Differ in Mass and Properties: The Diversity of Matter
This postulate emphasized the diversity within the atomic world. Dalton recognized that although atoms of the same element were identical, atoms of different elements possessed different masses and properties. This distinction was critical in understanding the variety of matter we observe in the universe. The differing properties of atoms explained the unique characteristics of various elements and their compounds. The differing masses formed the basis for later quantitative analysis in chemistry.
4. Atoms Combine in Simple, Whole-Number Ratios to Form Compounds: The Logic of Chemical Reactions
Dalton's fourth postulate addressed the formation of compounds. He proposed that atoms of different elements combine in simple, whole-number ratios to form compounds. This "law of definite proportions" is a cornerstone of chemical stoichiometry. For instance, the formation of water (H₂O) always involves two atoms of hydrogen for every one atom of oxygen. This simple ratio provided a powerful tool for understanding the quantitative aspects of chemical reactions. This postulate revolutionized the field of chemistry by providing a clear mathematical relationship between elements and their compounds. It laid the foundation for understanding how elements combined to create the vast array of substances in our world.
5. Atoms Cannot be Created, Destroyed, or Divided in Chemical Reactions: The Conservation of Matter
Dalton's final postulate spoke to the conservation of matter during chemical reactions. He stated that atoms are neither created nor destroyed in chemical processes; they simply rearrange themselves to form new compounds. This fundamental principle, often referred to as the law of conservation of mass, is crucial in balancing chemical equations and understanding the quantitative aspects of chemical transformations. This postulate underscored the immutable nature of atoms within chemical reactions, emphasizing that the total mass of reactants always equals the total mass of products.
The Impact of Dalton's Atomic Theory: A Paradigm Shift
Dalton's atomic theory represented a monumental leap forward in our understanding of the universe. Prior to its formulation, chemical reactions were largely described in a qualitative manner. Dalton's postulates provided a quantitative framework, paving the way for:
- Precise stoichiometric calculations: The law of definite proportions allowed chemists to accurately predict the amounts of reactants needed to produce a desired amount of product. This was a critical advance for quantitative chemical analysis and industrial processes.
- Development of the periodic table: The concept of atomic mass and the differing properties of atoms played a crucial role in the development of the periodic table, organizing elements based on their atomic properties and mass.
- Advancements in chemical nomenclature: Dalton's theory supported the standardization of chemical nomenclature, making it easier to communicate and understand chemical concepts across the scientific community.
- Foundation for later atomic models: Although Dalton's model was later refined, it provided a solid foundation for subsequent atomic models, such as the Rutherford model and the Bohr model. It introduced the fundamental concept of the atom as a fundamental building block of matter.
Limitations of Dalton's Atomic Theory: Unveiling the Subatomic World
Despite its revolutionary impact, Dalton's atomic theory possessed limitations that were revealed by later discoveries:
- Atoms are divisible: Subsequent discoveries, such as the discovery of electrons, protons, and neutrons, demonstrated that atoms are not indivisible but rather composed of subatomic particles. This contradicted Dalton's first postulate. The discovery of isotopes also contradicted his second postulate, showing that atoms of the same element could have slightly different masses.
- Isotopes: The existence of isotopes, atoms of the same element with different numbers of neutrons, demonstrated that not all atoms of a given element are identical in mass. This discovery challenged Dalton's assumption of identical atomic mass for all atoms of a single element.
- Molecular structure: Dalton's theory didn't address the arrangement of atoms within molecules. Later advancements in structural chemistry revealed the complex three-dimensional arrangements of atoms within molecules, influencing the physical and chemical properties of compounds.
- Nuclear reactions: Dalton's theory did not account for nuclear reactions, where atoms can be transformed into different elements through processes like radioactive decay. This was outside the scope of chemical reactions Dalton considered.
The Legacy of Dalton's Atomic Theory: A Continuing Influence
Despite its limitations, Dalton's atomic theory remains a landmark achievement in the history of science. It provided a fundamental framework for understanding matter, leading to significant advancements in chemistry and other scientific fields. Its postulates, though not entirely accurate, served as a springboard for more sophisticated atomic models and theories. The core concepts of atomic structure and the conservation of matter continue to be foundational principles in modern chemistry and physics.
Further Exploration of Atomic Theory: Beyond Dalton
The limitations of Dalton's model led to further exploration and refinement of atomic theory. Subsequent models, such as the Thomson model (plum pudding model), Rutherford model (nuclear model), and the Bohr model (planetary model), built upon Dalton's work, incorporating new discoveries about the subatomic world. These later models provided more accurate representations of atomic structure and behavior, accounting for phenomena that Dalton's theory could not explain. The development of quantum mechanics further refined our understanding of atomic behavior, allowing for a more accurate and comprehensive description of the atom and its interactions.
Conclusion: A Lasting Impact on Scientific Understanding
John Dalton's atomic theory, despite its limitations, stands as a cornerstone of modern chemistry. It introduced the fundamental concept of the atom as the basic building block of matter, revolutionizing our understanding of chemical reactions and paving the way for future advancements in atomic theory. While the specific postulates may have been refined or superseded, the overall impact of his theory on the scientific landscape remains undeniable. Dalton's legacy continues to inspire scientific inquiry and reminds us of the power of observation, experimentation, and the ongoing evolution of scientific understanding. His work represents a pivotal moment in the history of science, highlighting the iterative nature of scientific progress and the importance of building upon previous knowledge to achieve a more complete understanding of the world around us.
Latest Posts
Latest Posts
-
Describe How The Atoms In A Compound Are Held Together
Mar 17, 2025
-
How Is Absorbance Linked To Rate Of Reaction
Mar 17, 2025
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
-
Are Antiparalell Beta Sheets Mrore Stable
Mar 17, 2025
-
How Do I Solve Square Root Equations
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about According To Dalton's Atomic Theory An Atom Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
