All Neutral Atoms Of An Element Have The Same
Muz Play
Mar 16, 2025 · 5 min read
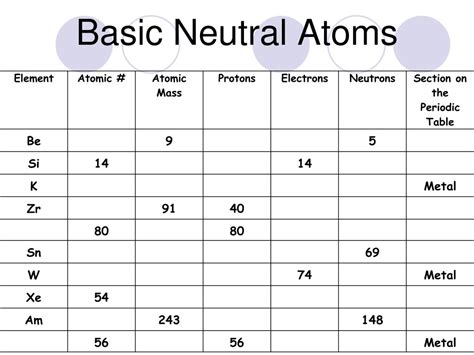
Table of Contents
All Neutral Atoms of an Element Have the Same: Exploring Atomic Structure and Isotopes
All neutral atoms of a given element have the same atomic number, which defines the element itself. This fundamental principle underpins our understanding of chemistry and the periodic table. Let's delve deeper into what this means and explore the nuances related to atomic structure, isotopes, and the implications for chemical behavior.
Understanding Atomic Number: The Defining Characteristic
The atomic number, represented by the symbol 'Z', signifies the number of protons found in the nucleus of an atom. Protons are positively charged subatomic particles, and they are crucial in determining an element's identity. Every atom of a specific element possesses the same number of protons. For instance, all hydrogen atoms have one proton (Z=1), all helium atoms have two protons (Z=2), and so on. This consistency in proton number is the cornerstone of the periodic table's organization, with elements arranged in ascending order of their atomic numbers.
The Role of Electrons and Neutrons
While the number of protons defines the element, the number of electrons and neutrons can vary. Electrons, negatively charged particles, orbit the nucleus and are equal in number to protons in a neutral atom. This balance of positive and negative charges ensures electrical neutrality.
Neutrons, which carry no charge, also reside within the nucleus. The number of neutrons can differ even within atoms of the same element, leading to the concept of isotopes.
Isotopes: Variations in Neutron Number
Isotopes are atoms of the same element that have the same number of protons but differ in the number of neutrons. This difference in neutron number alters the atom's mass, but not its chemical properties (significantly). The mass number (A) represents the total number of protons and neutrons in an atom's nucleus (A = Z + N, where N is the number of neutrons).
Different isotopes of the same element are typically denoted using the element's symbol, with the mass number as a superscript. For example:
- ¹²C (Carbon-12): 6 protons, 6 neutrons
- ¹³C (Carbon-13): 6 protons, 7 neutrons
- ¹⁴C (Carbon-14): 6 protons, 8 neutrons
All three are isotopes of carbon, distinguished by their mass numbers. They all have the same atomic number (Z=6), meaning they all have six protons, defining them as carbon atoms.
Implications of Isotopic Variations
The variations in neutron numbers have several implications:
-
Mass: Isotopes of the same element have different masses due to the different numbers of neutrons. This difference is significant in certain applications, such as mass spectrometry, which allows for the identification and quantification of different isotopes.
-
Stability: Some isotopes are stable, meaning their nuclei remain intact indefinitely. Others are radioactive, undergoing spontaneous decay and emitting particles or energy. This radioactive decay can have significant applications in medicine, dating techniques, and various industrial processes. For example, Carbon-14 is a radioactive isotope used in radiocarbon dating.
-
Chemical Properties: Despite differences in mass, isotopes of the same element generally exhibit the same chemical behavior. This is because chemical reactions primarily involve the interactions of electrons, which are determined by the number of protons (atomic number) and the electron configuration. However, slight differences in reaction rates (kinetic isotope effects) can sometimes be observed due to mass differences influencing vibrational frequencies and bond strengths.
The Periodic Table and Atomic Number
The periodic table is a powerful tool organizing elements based on their atomic number and resulting periodic properties. Elements are arranged in order of increasing atomic number, revealing recurring patterns in their physical and chemical characteristics. This arrangement reflects the underlying electron configurations and the consequent chemical behavior.
The columns (groups) represent elements with similar outer electron configurations, leading to similar chemical reactivity. The rows (periods) reflect the filling of electron shells, influencing atomic size and other properties. The organization based on atomic number provides a fundamental framework for understanding the relationships between elements and predicting their properties.
Beyond the Basics: Atomic Mass and Average Atomic Mass
While all neutral atoms of an element share the same atomic number, they may have different mass numbers due to isotopic variations. The concept of average atomic mass emerges due to the natural abundance of different isotopes. The average atomic mass, often simply referred to as atomic weight, is a weighted average of the masses of all naturally occurring isotopes of an element. This average reflects the relative abundance of each isotope in a naturally occurring sample.
For example, the average atomic mass of chlorine is approximately 35.45 amu, reflecting the natural abundance of its two main isotopes, ³⁵Cl and ³⁷Cl. This average value is crucial in various stoichiometric calculations and analytical chemistry applications.
Nuclear Chemistry and Isotopic Applications
The understanding of atomic number and isotopes is fundamental to nuclear chemistry, exploring the properties and reactions of atomic nuclei. Isotopes play vital roles in various applications, including:
-
Nuclear Medicine: Radioactive isotopes are used in diagnostic imaging (e.g., PET scans) and therapeutic treatments (e.g., radiotherapy).
-
Radioactive Dating: Radioactive isotopes with known half-lives are employed to determine the age of ancient artifacts and geological formations (e.g., carbon-14 dating, uranium-lead dating).
-
Industrial Tracers: Radioactive isotopes can be used to track the movement of materials in industrial processes.
-
Nuclear Power: Nuclear fission, involving the splitting of heavy atomic nuclei, is utilized in nuclear power plants to generate electricity.
-
Nuclear Fusion: Nuclear fusion, the merging of light atomic nuclei, is a potential source of clean energy, currently under intensive research and development.
Conclusion: The Defining Role of Atomic Number
In summary, while isotopes demonstrate variations in neutron number and consequently in mass, all neutral atoms of a given element share the same fundamental characteristic: their atomic number, which defines the number of protons in their nuclei. This critical parameter determines the element's identity, its position in the periodic table, and largely dictates its chemical behavior. Understanding this fundamental principle is crucial for comprehending atomic structure, chemical reactions, and various advanced applications involving isotopes in diverse fields such as medicine, geology, and energy production. The consistent atomic number for all neutral atoms of an element acts as a cornerstone of our understanding of matter and its interactions.
Latest Posts
Latest Posts
-
Describe How The Atoms In A Compound Are Held Together
Mar 17, 2025
-
How Is Absorbance Linked To Rate Of Reaction
Mar 17, 2025
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
-
Are Antiparalell Beta Sheets Mrore Stable
Mar 17, 2025
-
How Do I Solve Square Root Equations
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about All Neutral Atoms Of An Element Have The Same . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
