An Element's Atomic Number Is Equal To Its Number Of
Muz Play
Mar 15, 2025 · 7 min read
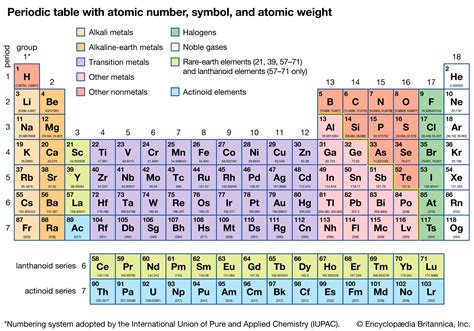
Table of Contents
An Element's Atomic Number is Equal to its Number of Protons: A Deep Dive into Atomic Structure
Understanding the fundamental building blocks of matter is crucial in chemistry and physics. At the heart of this understanding lies the atom, and within the atom, the concept of atomic number holds paramount importance. This article delves deep into the meaning of atomic number, exploring its relationship to protons, its role in defining elements, and its implications in various scientific fields.
What is Atomic Number?
The atomic number of an element is the number of protons found in the nucleus of each of its atoms. This is a fundamental characteristic that uniquely identifies each element on the periodic table. It's a whole number, never a fraction or decimal, and is represented by the symbol Z. For example, the atomic number of hydrogen (Z = 1) means that every hydrogen atom contains one proton in its nucleus. Similarly, oxygen (Z = 8) has eight protons, and uranium (Z = 92) possesses ninety-two protons.
The Significance of Protons
Protons are positively charged subatomic particles located within the atom's nucleus. They are significantly more massive than electrons (negatively charged particles orbiting the nucleus) and, along with neutrons (neutral particles in the nucleus), determine the mass of an atom. However, it's the number of protons, not neutrons or electrons, that defines the element itself.
Why Protons, Not Neutrons or Electrons?
While neutrons contribute to an atom's mass (forming isotopes), the number of neutrons can vary within the same element. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. For example, carbon-12 and carbon-14 are both isotopes of carbon, differing in the number of neutrons (6 and 8, respectively) but having the same number of protons (6).
Electrons, on the other hand, determine an atom's chemical properties and its ability to form bonds with other atoms. While the number of electrons can change (forming ions), the number of protons remains constant for a given element. The element's identity is intrinsically linked to its nuclear charge – the total positive charge from its protons. This is why atomic number, representing the proton count, is the definitive characteristic of an element.
The Periodic Table and Atomic Number
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number. Elements are arranged in increasing order of atomic number, reflecting a systematic progression in their electronic structure and, consequently, their chemical properties. This arrangement allows scientists to predict and understand trends in reactivity, electronegativity, and other atomic characteristics.
The table is structured into rows (periods) and columns (groups). Elements within the same group share similar chemical properties due to similar arrangements of their outermost electrons (valence electrons). The atomic number provides the key to understanding this arrangement and the resultant chemical behavior. Understanding atomic number is therefore crucial for interpreting the periodic table and predicting the behavior of elements.
Atomic Number and Isotopes: A Deeper Look
As mentioned earlier, isotopes are atoms of the same element with the same atomic number (same number of protons) but different numbers of neutrons. This difference in neutron number results in different mass numbers. The mass number (A) is the sum of protons and neutrons in an atom's nucleus.
Isotopes of an element exhibit similar chemical properties because they have the same number of electrons and thus the same electronic configuration. However, their physical properties, especially mass, can vary due to the difference in neutron number. Some isotopes are stable, while others are radioactive, meaning they undergo spontaneous decay, emitting particles and energy. The study of isotopes has widespread applications in various fields, including:
- Radioactive dating: Utilizing radioactive isotopes with known half-lives to determine the age of materials.
- Medical imaging and treatment: Employing radioactive isotopes for diagnostic scans and cancer therapy.
- Tracers in scientific research: Utilizing radioactive isotopes to track the movement of substances in biological and chemical systems.
Understanding the concept of isotopes and their relationship to atomic number is essential in these applications.
Atomic Number and Nuclear Chemistry
Atomic number plays a crucial role in understanding nuclear reactions. Nuclear reactions involve changes in the nucleus of an atom, often resulting in changes in the atomic number and thus the element itself.
Nuclear fission: The splitting of a heavy atomic nucleus into two lighter nuclei, often releasing a significant amount of energy. The products of fission typically have different atomic numbers than the original nucleus.
Nuclear fusion: The combining of two light atomic nuclei to form a heavier nucleus, also releasing substantial energy. The resulting nucleus has a different atomic number than the original nuclei.
Radioactive decay: The spontaneous emission of particles or energy from an unstable atomic nucleus. Different types of decay (alpha, beta, gamma) lead to changes in the atomic number and/or mass number of the nucleus. Understanding the changes in atomic number during these processes is essential for predicting the products of nuclear reactions and assessing their potential impact.
Atomic Number and Spectroscopic Analysis
The atomic number of an element is intrinsically linked to its electronic structure and energy levels. When an atom absorbs energy, its electrons can jump to higher energy levels. When these excited electrons return to their ground state, they emit energy in the form of light. This emitted light has a specific wavelength or frequency, characteristic of the element. This principle forms the basis of spectroscopic analysis, a powerful technique used to identify and quantify elements in a sample. Each element has a unique emission spectrum, a "fingerprint" based on its atomic number and electronic structure. Spectroscopic analysis is extensively used in various fields, including:
- Astronomy: Identifying the composition of stars and other celestial objects.
- Environmental monitoring: Analyzing pollutants and contaminants in air, water, and soil.
- Forensic science: Identifying substances and materials at crime scenes.
The analysis of these spectra heavily relies on the unique relationship between an element's atomic number and its characteristic emission spectrum.
Atomic Number and Chemical Bonding
The atomic number directly influences the number of valence electrons an atom possesses. Valence electrons are the electrons in the outermost shell of an atom and are primarily responsible for chemical bonding. Atoms tend to interact with each other to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons.
The number of valence electrons, determined by the atomic number and electronic structure, dictates the type of bonds an atom can form (ionic, covalent, metallic) and the number of bonds it can form. This determines the chemical properties of the element and its reactivity with other elements. Understanding the connection between atomic number, valence electrons, and chemical bonding is essential for understanding chemical reactions and predicting the properties of compounds.
Atomic Number and Its Practical Applications
The concept of atomic number extends far beyond theoretical chemistry and physics. Its practical applications are vast and impact numerous fields:
- Material Science: Designing new materials with specific properties based on the atomic composition and bonding characteristics.
- Nuclear Medicine: Developing radioactive isotopes for diagnostic and therapeutic purposes.
- Nuclear Energy: Utilizing nuclear fission and fusion reactions to generate energy.
- Environmental Science: Monitoring and remediating environmental pollution.
- Analytical Chemistry: Identifying and quantifying elements in various samples.
In each of these applications, the fundamental understanding of atomic number and its relationship to the properties of elements forms the bedrock of scientific progress.
Conclusion
The atomic number of an element, representing the number of protons in its nucleus, is a fundamental concept that underlies our understanding of matter and its behavior. It uniquely identifies each element, dictates its chemical properties, influences its nuclear reactions, and plays a crucial role in various spectroscopic and analytical techniques. The significance of atomic number extends far beyond the realm of theoretical science, finding widespread application in diverse fields, showcasing its practical importance in our daily lives and technological advancements. A deep understanding of atomic number is, therefore, essential for anyone seeking to comprehend the intricacies of the physical world and its applications. Further exploration into isotopic variations, nuclear processes, and spectroscopic techniques will further enhance this understanding and provide a more comprehensive appreciation of this fundamental aspect of atomic structure.
Latest Posts
Latest Posts
-
How To Determine Shape Of A Molecule
Mar 15, 2025
-
Is A Negative Delta H Exothermic
Mar 15, 2025
-
What Is The Main Purpose Of Photosynthesis
Mar 15, 2025
-
The Shape Of Protein Is Determined By
Mar 15, 2025
-
Are Homologous Chromosomes Present In Mitosis
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about An Element's Atomic Number Is Equal To Its Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
