Are Carboxylic Acids Soluble In Water
Muz Play
Mar 21, 2025 · 6 min read
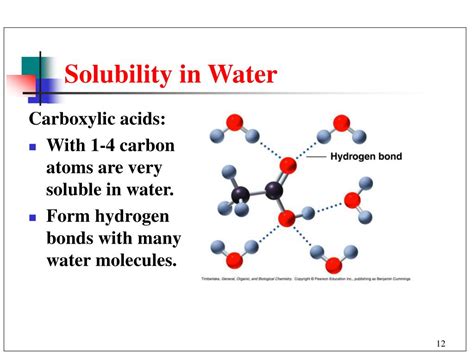
Table of Contents
Are Carboxylic Acids Soluble in Water? A Deep Dive into Solubility
Carboxylic acids, characterized by their -COOH functional group, are a ubiquitous class of organic compounds found in numerous natural products and synthetic materials. Understanding their solubility in water is crucial in various fields, from biochemistry and pharmacology to environmental science and industrial chemistry. While a simple answer might seem straightforward, the reality is more nuanced, requiring a deeper exploration of the interplay between molecular structure, intermolecular forces, and the nature of the solvent. This article delves into the intricacies of carboxylic acid solubility in water, examining the factors that influence it and providing a comprehensive understanding of this fundamental concept.
The Role of Polarity and Hydrogen Bonding
The solubility of any compound in water is fundamentally governed by the principle "like dissolves like." Water, a highly polar molecule, readily dissolves polar and ionic substances. Carboxylic acids possess a polar carbonyl group (C=O) and a highly polar hydroxyl group (-OH). This combination makes them significantly more polar than many other organic compounds. The presence of these polar groups enables strong interactions with water molecules through hydrogen bonding.
Hydrogen Bonding: The Key Interaction
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen in the -OH and C=O groups) is attracted to a lone pair of electrons on another electronegative atom in a different molecule. In the case of carboxylic acids in water, hydrogen bonds form between the oxygen atoms of the carboxyl group (-COOH) and the hydrogen atoms of water molecules. Similarly, the hydrogen atom of the -OH group in the carboxylic acid can form a hydrogen bond with the oxygen atom of a water molecule. This extensive network of hydrogen bonds is the primary driving force behind the solubility of many carboxylic acids in water.
Influence of the Carbon Chain Length
However, the story doesn't end with polarity and hydrogen bonding. The size and structure of the hydrocarbon chain attached to the carboxyl group also significantly impact solubility. As the length of the hydrocarbon chain increases, the nonpolar character of the molecule dominates. This hydrophobic (water-repelling) nature competes with the hydrophilic (water-attracting) nature of the carboxyl group.
-
Short-chain carboxylic acids: Carboxylic acids with short alkyl chains (e.g., formic acid, acetic acid, propionic acid) are generally soluble in water because the polar carboxyl group's influence outweighs the hydrophobic effect of the small hydrocarbon chain. The hydrogen bonding interactions with water molecules are strong enough to overcome the hydrophobic interactions.
-
Long-chain carboxylic acids: As the alkyl chain length increases (e.g., butanoic acid, pentanoic acid, and beyond), the hydrophobic interactions become increasingly significant. The long hydrocarbon tail tends to cluster together, minimizing its contact with water. This results in a decrease in solubility. These longer-chain carboxylic acids are often less soluble or even insoluble in water. They may exhibit higher solubility in organic solvents.
Factors Affecting Carboxylic Acid Solubility
Beyond chain length, several other factors can influence the solubility of carboxylic acids in water:
Branching of the Carbon Chain
Branching of the hydrocarbon chain can subtly affect solubility. Branched-chain carboxylic acids often exhibit slightly higher solubility than their straight-chain counterparts of the same molecular weight. This is because branching reduces the effective surface area of the hydrophobic portion of the molecule, leading to less interaction with other hydrocarbon chains and therefore a greater tendency to interact with water.
Presence of Other Functional Groups
The presence of additional functional groups on the hydrocarbon chain can significantly alter solubility. For instance, the introduction of hydroxyl (-OH) or amino (-NH2) groups increases the polarity of the molecule and enhances its solubility in water. Conversely, the presence of halogen atoms (Cl, Br, I) usually decreases solubility due to their electron-withdrawing effects, reducing the polarity of the carboxyl group.
Temperature
Temperature also plays a role in solubility. Generally, the solubility of carboxylic acids in water increases with increasing temperature. This is because higher temperatures provide more kinetic energy, facilitating the breaking of hydrogen bonds and allowing better dispersion of the carboxylic acid molecules within the water.
pH of the Solution
The pH of the solution significantly impacts the solubility of carboxylic acids. Carboxylic acids are weak acids, meaning they partially dissociate in water to form carboxylate ions (-COO⁻) and hydronium ions (H₃O⁺). At low pH (acidic conditions), the carboxylic acid exists predominantly in its undissociated form, which is less soluble in water due to the hydrophobic character of the hydrocarbon chain. At higher pH (alkaline conditions), the carboxylic acid deprotonates, forming the carboxylate ion, which is much more soluble in water due to its increased polarity and the ability to engage in stronger ionic interactions.
Practical Applications and Implications
The solubility of carboxylic acids in water has numerous practical implications across various scientific and industrial domains:
Pharmaceutical Sciences
The solubility of drug molecules, many of which are carboxylic acids, directly impacts their bioavailability and efficacy. If a drug is poorly soluble, it may not be absorbed effectively from the gastrointestinal tract, leading to reduced therapeutic effects. Pharmaceutical scientists use various strategies to enhance the solubility of drug molecules, including salt formation, the use of co-solvents, and the design of prodrugs.
Environmental Science
The solubility of carboxylic acids in water is crucial in understanding their environmental fate and transport. Carboxylic acids found in pollutants and industrial waste can dissolve in water and be transported through aquatic systems. Their solubility influences their potential for bioaccumulation in organisms and their impact on the environment.
Food Science
Many organic acids found in foods, such as citric acid, acetic acid, and lactic acid, are carboxylic acids. Their solubility plays a significant role in their taste, texture, and preservation properties.
Conclusion
The solubility of carboxylic acids in water is not a simple yes or no answer. It's a complex interplay between the polar nature of the carboxyl group, the hydrophobic nature of the hydrocarbon chain, and a variety of other factors including temperature, pH, and the presence of other functional groups. Understanding these factors is essential in numerous fields, from designing effective drug delivery systems to predicting the environmental impact of pollutants. While short-chain carboxylic acids are generally readily soluble, the solubility decreases significantly as the length of the hydrocarbon chain increases. Furthermore, manipulation of pH can dramatically affect solubility, offering valuable tools for chemical processing and pharmaceutical development. The detailed analysis presented here provides a strong foundation for comprehending this key concept in organic chemistry and its far-reaching consequences.
Latest Posts
Latest Posts
-
Which Is The Best Definition Of Directional Selection
Mar 22, 2025
-
How To Find The Boundaries In Statistics
Mar 22, 2025
-
How Many Lone Pairs Does H2o Have
Mar 22, 2025
-
Second Formulation Of The Categorical Imperative
Mar 22, 2025
-
Calculate The Ph At The Equivalence Point
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Are Carboxylic Acids Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
