Are Covalent Bonds Stronger Than Ionic Bonds
Muz Play
Mar 15, 2025 · 6 min read
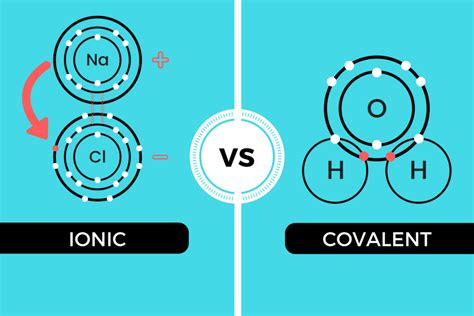
Table of Contents
Are Covalent Bonds Stronger Than Ionic Bonds? A Deep Dive into Chemical Bonding
The question of whether covalent bonds are stronger than ionic bonds isn't a simple yes or no. The strength of a chemical bond is a complex issue dependent on several factors, making a direct comparison challenging. While generalizations can be made, the reality is often more nuanced. This article will delve deep into the nature of both covalent and ionic bonds, exploring their strengths and weaknesses, and examining the circumstances under which one might be considered "stronger" than the other.
Understanding Chemical Bonds: The Foundation of Matter
Before comparing covalent and ionic bonds, let's establish a foundational understanding of what they are. Chemical bonds are the forces that hold atoms together to form molecules and compounds. These forces arise from the electrostatic interactions between charged particles – primarily electrons and protons. The nature of these interactions determines the type of bond formed.
Ionic Bonds: The Dance of Ions
Ionic bonds are formed through the electrostatic attraction between oppositely charged ions. This occurs when one atom donates one or more electrons to another atom, creating a cation (positively charged ion) and an anion (negatively charged ion). The strong Coulombic attraction between these ions holds them together in a crystal lattice structure.
Key characteristics of ionic bonds:
- Electron transfer: A complete transfer of electrons occurs between atoms.
- Electrostatic attraction: The bond is formed by the attraction between oppositely charged ions.
- High melting and boiling points: The strong electrostatic forces require significant energy to overcome, leading to high melting and boiling points.
- Crystalline structure: Ionic compounds typically form crystalline solids with a regular, repeating arrangement of ions.
- Conductivity in solution: Ionic compounds conduct electricity when dissolved in water or molten, as the ions become mobile.
- Brittleness: Ionic crystals are brittle because the displacement of ions can lead to repulsion between like charges, causing the crystal to fracture.
Covalent Bonds: Sharing is Caring
Covalent bonds, on the other hand, are formed by the sharing of electrons between atoms. This sharing allows both atoms to achieve a more stable electron configuration, typically a full outer electron shell. The shared electrons are attracted to the nuclei of both atoms, holding them together.
Key characteristics of covalent bonds:
- Electron sharing: Electrons are shared between atoms, not transferred.
- Overlapping orbitals: The shared electrons occupy regions of space called molecular orbitals, formed by the overlap of atomic orbitals.
- Variable bond strengths: Covalent bond strength varies depending on the atoms involved and the number of shared electron pairs (single, double, or triple bonds).
- Lower melting and boiling points (generally): Covalent compounds generally have lower melting and boiling points than ionic compounds because the intermolecular forces are weaker than the strong electrostatic forces in ionic compounds. However, exceptions exist, particularly with network covalent solids like diamond.
- Poor conductivity: Covalent compounds generally do not conduct electricity because they do not have free-moving charged particles.
- Variety of structures: Covalent compounds can exist as gases, liquids, or solids, and can form diverse structures ranging from simple diatomic molecules to complex polymers.
Comparing Bond Strengths: A Multifaceted Issue
Determining whether covalent or ionic bonds are "stronger" requires considering various factors:
-
Bond energy: Bond energy is the amount of energy required to break a bond. This is a crucial measure of bond strength. Ionic bond energies are often high, reflecting the strong electrostatic attraction between ions. Covalent bond energies also vary widely, depending on factors like bond order (single, double, triple) and the electronegativity difference between atoms. Generally, triple bonds are stronger than double bonds, which are stronger than single bonds.
-
Bond length: Bond length is the distance between the nuclei of two bonded atoms. Shorter bond lengths generally indicate stronger bonds. Ionic bond lengths are typically longer than covalent bond lengths because of the larger size of ions compared to atoms.
-
Electronegativity: Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A large electronegativity difference between atoms leads to more ionic character in a bond, while a small difference results in a more covalent character. However, even bonds considered "covalent" can exhibit some degree of ionic character, and vice versa. This is reflected in the concept of bond polarity.
-
Intermolecular forces: In covalent compounds, the strength of intermolecular forces (like van der Waals forces, dipole-dipole interactions, and hydrogen bonding) also significantly influences the overall properties of the substance. These forces are much weaker than ionic or covalent bonds themselves but play a crucial role in the physical state and properties of molecules.
The complication: A highly polar covalent bond might exhibit a bond strength comparable to a weak ionic bond, and a strong covalent triple bond could be significantly stronger than a weak ionic bond. Thus, a blanket statement about which bond type is always stronger is inaccurate.
Examples Illustrating the Nuances
Let's analyze some specific examples to demonstrate the complexities involved:
-
Sodium chloride (NaCl): A classic example of an ionic compound, NaCl has a strong ionic bond due to the large electronegativity difference between sodium and chlorine. It has a high melting point (801°C) reflecting this strong bond.
-
Diamond (C): Diamond is a network covalent solid where carbon atoms are bonded together in a strong tetrahedral network via covalent bonds. The strength of these bonds results in diamond's extreme hardness and high melting point (3550°C). This example highlights that covalent bonds can be incredibly strong, surpassing many ionic bonds.
-
Hydrogen fluoride (HF): HF has a highly polar covalent bond due to the significant electronegativity difference between hydrogen and fluorine. While it's still considered covalent, the high polarity results in strong intermolecular hydrogen bonding, leading to a higher boiling point than expected for its molecular weight.
-
Nitrogen gas (N₂): Nitrogen gas exists as diatomic molecules (N₂) with a very strong triple covalent bond. The exceptionally high bond energy makes it very unreactive.
Conclusion: Context Matters
The question of whether covalent or ionic bonds are stronger is ultimately context-dependent. While ionic bonds generally exhibit high bond energies due to strong electrostatic attraction, covalent bonds, particularly multiple bonds and those in network solids, can exhibit comparable or even greater strengths. The electronegativity difference between atoms, bond order, and the presence of intermolecular forces all play critical roles in determining the overall strength and properties of a substance. Therefore, a definitive answer to the question is not possible without considering the specific chemical species involved. The crucial takeaway is that understanding the nuances of both ionic and covalent bonding is essential for comprehending the vast array of chemical properties observed in the world around us.
Latest Posts
Latest Posts
-
Acids And Bases Cannot Mix Together
Mar 15, 2025
-
Linear Programming Do Not Find Minimum Or Maximum
Mar 15, 2025
-
The Change Rate Of Angular Momentum Equals To
Mar 15, 2025
-
Difference Between Tlc And Column Chromatography
Mar 15, 2025
-
Energy Required To Remove An Electron From A Gaseous Atom
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Are Covalent Bonds Stronger Than Ionic Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
