Energy Required To Remove An Electron From A Gaseous Atom
Muz Play
Mar 15, 2025 · 5 min read
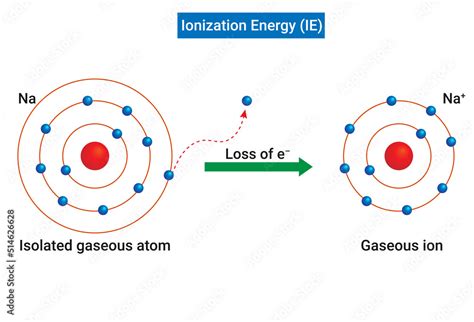
Table of Contents
The Energy Required to Remove an Electron from a Gaseous Atom: Ionization Energy Explained
The energy required to remove an electron from a gaseous atom is a fundamental concept in chemistry and physics, known as ionization energy (IE). Understanding ionization energy is crucial for comprehending atomic structure, chemical bonding, and the behavior of matter in various states. This comprehensive article delves into the intricacies of ionization energy, exploring its definition, trends across the periodic table, factors influencing its value, and its applications in diverse fields.
What is Ionization Energy?
Ionization energy, also referred to as ionization potential, is the minimum amount of energy required to remove the most loosely bound electron from a neutral gaseous atom in its ground state. This process transforms the neutral atom into a positively charged ion (cation). The removal of subsequent electrons requires progressively higher amounts of energy, leading to the concept of successive ionization energies.
The process can be represented by the following equation:
X(g) + energy → X⁺(g) + e⁻
where:
- X(g) represents a neutral gaseous atom
- X⁺(g) represents the resulting cation
- e⁻ represents the removed electron
The energy required is typically expressed in kilojoules per mole (kJ/mol) or electron volts (eV).
Factors Affecting Ionization Energy
Several factors influence the magnitude of ionization energy:
1. Effective Nuclear Charge (Z<sub>eff</sub>):
The effective nuclear charge is the net positive charge experienced by an electron in an atom. It's the difference between the actual nuclear charge (number of protons) and the shielding effect of inner electrons. A higher effective nuclear charge results in a stronger attraction between the nucleus and the valence electrons, leading to a higher ionization energy.
2. Atomic Radius:
The distance between the nucleus and the valence electrons is crucial. A larger atomic radius implies a weaker electrostatic attraction between the nucleus and the valence electron, resulting in a lower ionization energy. Conversely, smaller atoms exhibit higher ionization energies.
3. Shielding Effect:
Inner electrons partially shield the valence electrons from the full positive charge of the nucleus. Increased shielding reduces the effective nuclear charge experienced by valence electrons, lowering the ionization energy. This effect is particularly pronounced in atoms with multiple electron shells.
4. Electron Configuration:
The electron configuration of an atom significantly impacts its ionization energy. Electrons in filled or half-filled subshells are relatively stable due to electron-electron repulsions and exchange energy, requiring more energy to remove. Electrons in partially filled subshells are less stable and easier to remove.
5. Penetration Effect:
Certain orbitals penetrate closer to the nucleus than others. Electrons in orbitals with higher penetration experience a stronger effective nuclear charge, leading to higher ionization energy. For example, s orbitals penetrate more effectively than p orbitals, which in turn penetrate more effectively than d orbitals.
Trends in Ionization Energy Across the Periodic Table
Ionization energy exhibits predictable trends across the periodic table, reflecting the interplay of the factors discussed above:
1. Across a Period (Left to Right):
As you move across a period from left to right, the atomic radius generally decreases, and the effective nuclear charge increases. This leads to a general increase in ionization energy. However, slight irregularities can occur due to variations in electron configurations and electron-electron repulsions.
2. Down a Group (Top to Bottom):
Moving down a group, the atomic radius increases significantly, and the shielding effect becomes more pronounced. This results in a decrease in ionization energy as the valence electrons are further from the nucleus and experience a weaker effective nuclear charge.
Successive Ionization Energies
Removing subsequent electrons from an ion requires progressively more energy. This is because the remaining electrons experience a higher effective nuclear charge as the number of electrons decreases. The difference between successive ionization energies provides insights into the electronic structure of the atom. Large jumps in ionization energy often indicate the completion of a subshell.
For example, consider the successive ionization energies of sodium (Na):
- First ionization energy: Relatively low due to the removal of a single valence electron.
- Second ionization energy: Significantly higher because the electron is removed from a filled inner shell, experiencing a much stronger effective nuclear charge.
- Subsequent ionization energies: Continue to increase dramatically.
Applications of Ionization Energy
Ionization energy plays a vital role in several scientific and technological applications:
- Spectroscopy: Analyzing the energy of photons absorbed or emitted during ionization provides valuable information about the electronic structure of atoms and molecules.
- Mass Spectrometry: Ionization is a fundamental step in mass spectrometry, a technique used to determine the mass-to-charge ratio of ions, aiding in the identification and quantification of molecules.
- Chemical Bonding: Ionization energies help predict the reactivity and bonding behavior of elements. Elements with low ionization energies tend to lose electrons easily, forming cations, while elements with high ionization energies tend to gain electrons, forming anions.
- Plasma Physics: Ionization is crucial in generating and controlling plasmas, which have numerous applications in areas such as lighting, semiconductor manufacturing, and fusion energy research.
- Photoelectron Spectroscopy (PES): This technique measures the kinetic energy of electrons emitted when atoms or molecules are irradiated with high-energy photons. Analyzing this data reveals detailed information about the energy levels of electrons in the atom or molecule, directly related to ionization energies.
- Understanding Stellar Processes: Ionization energies are essential in astrophysics for understanding the processes occurring in stars, including nuclear fusion and the formation of elements.
Conclusion
Ionization energy is a cornerstone concept in atomic physics and chemistry, providing fundamental insights into the structure and behavior of matter. Its value depends on several interrelated factors, including effective nuclear charge, atomic radius, shielding effect, and electron configuration. The predictable trends observed across the periodic table, along with the concept of successive ionization energies, provide a powerful tool for understanding chemical reactivity and electronic structure. Moreover, ionization energy finds diverse applications in various fields, ranging from spectroscopy and mass spectrometry to plasma physics and astrophysics, highlighting its importance in modern science and technology. Further research and advancements in related techniques will continue to expand our understanding and applications of this crucial parameter. The exploration of ionization energy remains a dynamic and essential area of scientific investigation, promising further discoveries and technological innovations in the years to come.
Latest Posts
Latest Posts
-
Is Density Physical Or Chemical Property
Mar 15, 2025
-
A Process That Does Not Require Oxygen
Mar 15, 2025
-
Is Blood Clotting Positive Or Negative Feedback
Mar 15, 2025
-
Difference Between Nuclear And Chemical Reactions
Mar 15, 2025
-
Good Conductor Of Heat Metal Nonmetal Or Metalloid
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Energy Required To Remove An Electron From A Gaseous Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
