Good Conductor Of Heat Metal Nonmetal Or Metalloid
Muz Play
Mar 15, 2025 · 6 min read
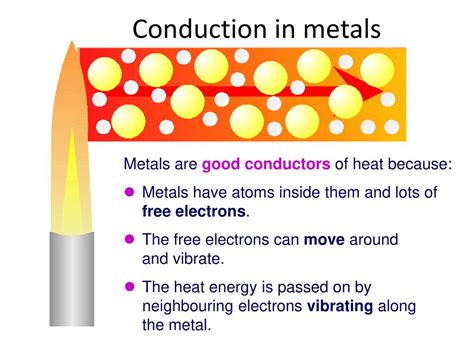
Table of Contents
Good Conductor of Heat: Metal, Nonmetal, or Metalloid?
Understanding the thermal conductivity of materials is crucial in various fields, from engineering and manufacturing to cooking and electronics. The ability of a substance to transfer heat is a fundamental property, and the classification of materials as metals, nonmetals, or metalloids significantly impacts their heat transfer capabilities. This article delves into the intricacies of heat conduction, exploring why metals generally excel as heat conductors compared to nonmetals and metalloids. We'll examine the underlying atomic structures and electron behavior responsible for these differences.
The Science of Heat Conduction
Heat conduction is the process by which thermal energy is transferred through a material from a region of higher temperature to a region of lower temperature. This transfer occurs due to the vibrational motion of atoms and molecules and, crucially, the movement of electrons. The efficiency of this process is quantified by a material's thermal conductivity, typically measured in Watts per meter-Kelvin (W/m·K). A higher thermal conductivity value indicates a more efficient heat conductor.
The Role of Electrons
The primary driver behind the exceptional thermal conductivity of many metals lies in their unique electronic structure. Metals possess a "sea" of delocalized electrons—electrons not bound to any particular atom but free to move throughout the entire metallic lattice. These free electrons are highly mobile and readily absorb thermal energy. This absorbed energy is then rapidly transferred throughout the material as the electrons collide with other atoms and electrons, efficiently distributing the heat.
Lattice Vibrations (Phonons)
While electron movement is dominant in metals, another mechanism contributes to heat conduction in all materials: lattice vibrations. Atoms in a solid are not stationary; they vibrate around their equilibrium positions. These vibrations, quantized as phonons, can also transport thermal energy. However, the efficiency of phonon-mediated heat transfer is generally lower than electron-mediated transfer, especially in metals.
Metals: The Champions of Heat Conduction
Metals, with their characteristic sea of delocalized electrons, are undeniably the best conductors of heat. This high thermal conductivity is a direct consequence of the mobility of their valence electrons. The free electrons can quickly and efficiently transfer kinetic energy (heat) throughout the metallic structure.
Examples of Excellent Metallic Heat Conductors:
-
Copper (Cu): Copper boasts an exceptionally high thermal conductivity, making it a material of choice in applications requiring efficient heat dissipation, such as heat exchangers, cookware, and electrical wiring.
-
Aluminum (Al): Aluminum, lighter than copper, offers a good balance between thermal conductivity and cost-effectiveness, frequently used in heat sinks, automotive parts, and construction materials.
-
Silver (Ag): While expensive, silver possesses the highest thermal conductivity of all metals, making it ideal for specialized applications where superior heat transfer is paramount, albeit at a premium cost.
-
Gold (Au): Gold also exhibits high thermal conductivity, often utilized in electronics and specialized applications requiring both excellent conductivity and corrosion resistance.
Factors Affecting Metallic Thermal Conductivity:
Several factors influence the thermal conductivity of metals:
-
Purity: Impurities in the metal lattice disrupt the flow of electrons, reducing thermal conductivity. Higher purity generally translates to higher conductivity.
-
Temperature: Thermal conductivity usually decreases with increasing temperature. This is because increased atomic vibrations interfere with electron movement.
-
Crystal Structure: The arrangement of atoms in the metal lattice affects electron mobility and thus, thermal conductivity. A well-ordered lattice facilitates efficient electron flow.
Nonmetals: Poor Heat Conductors
In stark contrast to metals, nonmetals are generally poor conductors of heat. This is because nonmetals lack the sea of delocalized electrons that facilitate efficient heat transfer in metals. The electrons in nonmetals are tightly bound to their respective atoms, restricting their mobility. Consequently, heat transfer relies primarily on the less efficient phonon mechanism.
Examples of Nonmetallic Poor Heat Conductors:
-
Wood: Wood is a poor heat conductor, a property that makes it suitable for applications requiring insulation, such as in construction.
-
Plastics: Most plastics are excellent insulators, meaning they resist heat transfer. This property is widely exploited in electrical insulation and various household applications.
-
Glass: Glass is a relatively poor heat conductor, making it a suitable material for insulation purposes and cookware handles.
-
Rubber: Rubber is another excellent insulator, often used in applications requiring heat resistance and insulation.
Factors Affecting Nonmetal Thermal Conductivity:
The thermal conductivity of nonmetals is influenced by:
-
Molecular Structure: The arrangement and bonding of atoms and molecules significantly affect the efficiency of phonon-mediated heat transfer.
-
Density: Denser materials tend to have slightly higher thermal conductivity due to increased atomic interactions.
-
Temperature: Similar to metals, the thermal conductivity of nonmetals can change with temperature, though the effect might be less pronounced.
Metalloids: A Middle Ground
Metalloids occupy a position intermediate between metals and nonmetals in terms of their properties. Their thermal conductivity is generally lower than that of metals but higher than that of most nonmetals. The behavior of electrons in metalloids is more complex than in either metals or nonmetals, leading to a range of thermal conductivities.
Examples of Metalloids and Their Thermal Conductivity:
-
Silicon (Si): Silicon, a crucial semiconductor material, exhibits a moderate thermal conductivity, which is crucial in managing heat generation in electronic devices.
-
Germanium (Ge): Similar to silicon, germanium has a relatively moderate thermal conductivity and is used in some semiconductor applications.
-
Arsenic (As): Arsenic's thermal conductivity is relatively low compared to metals but higher than many nonmetals.
Factors Affecting Metalloid Thermal Conductivity:
The thermal conductivity of metalloids is influenced by factors similar to those affecting metals and nonmetals, including:
-
Doping: Introducing impurities (doping) can significantly alter the electronic structure and thermal conductivity of metalloids.
-
Crystalline Structure: The crystal structure and its perfection affect the transport of both electrons and phonons.
-
Temperature: Temperature significantly influences the thermal conductivity of metalloids, as it affects the carrier mobility.
Applications Based on Thermal Conductivity
The differing thermal conductivities of metals, nonmetals, and metalloids lead to a wide range of applications across diverse fields:
-
Heat Exchangers: High thermal conductivity metals such as copper and aluminum are essential in heat exchangers used in various industrial processes, power plants, and HVAC systems.
-
Cooking Utensils: Copper and aluminum are favored for cookware due to their excellent heat conduction, ensuring even heating.
-
Electronics: The thermal management of electronic devices relies heavily on materials with appropriate thermal conductivities. Heat sinks made of high thermal conductivity metals are crucial in dissipating heat generated by electronic components. Silicon's moderate thermal conductivity is essential for managing heat in microchips.
-
Building Insulation: Nonmetallic materials with low thermal conductivities are used extensively in building insulation to reduce energy loss and improve thermal comfort.
-
Automotive Parts: Aluminum's combination of lightweight and high thermal conductivity makes it suitable for various automotive components, reducing vehicle weight and improving fuel efficiency.
Conclusion
The thermal conductivity of a material is a critical property determined by its atomic structure and electron behavior. Metals, with their sea of delocalized electrons, are exceptionally good heat conductors. Nonmetals, lacking this electron mobility, are generally poor conductors. Metalloids occupy an intermediate position. Understanding these differences is vital for selecting appropriate materials for various applications, ranging from efficient heat dissipation in electronics to effective thermal insulation in buildings. Further research and development in materials science continue to explore new materials with tailored thermal conductivities to meet the ever-evolving demands of technology and various industries.
Latest Posts
Latest Posts
-
Factors That Influence The Elasticity Of Supply
Mar 17, 2025
-
Describe How The Atoms In A Compound Are Held Together
Mar 17, 2025
-
How Is Absorbance Linked To Rate Of Reaction
Mar 17, 2025
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
-
Are Antiparalell Beta Sheets Mrore Stable
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Good Conductor Of Heat Metal Nonmetal Or Metalloid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
