Difference Between Nuclear And Chemical Reactions
Muz Play
Mar 15, 2025 · 6 min read
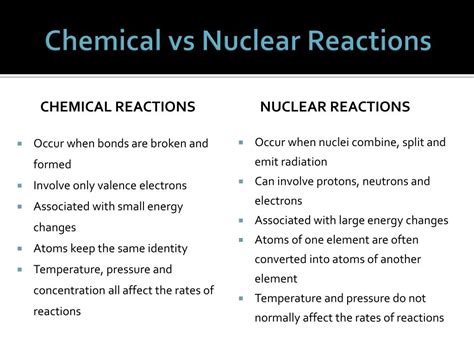
Table of Contents
Delving Deep: The Fundamental Differences Between Nuclear and Chemical Reactions
Understanding the difference between nuclear and chemical reactions is crucial for comprehending the world around us, from the energy that powers our homes to the processes that shape the universe. While both involve transformations of matter, they operate on fundamentally different scales and mechanisms. This article will explore these differences in detail, highlighting the key distinctions in terms of energy changes, particle involvement, reaction rates, and the types of reactions themselves.
The Scale of Change: Subatomic vs. Molecular
The most fundamental difference lies in the scale at which these reactions occur. Chemical reactions involve the rearrangement of atoms within molecules. Atoms retain their identity—the same number of protons defines the element—but their bonds are broken and reformed, creating new molecules with different properties. Think of baking a cake: flour, sugar, eggs, and butter are combined, and chemical reactions transform these ingredients into a delicious cake, but the atoms themselves remain unchanged.
Nuclear reactions, on the other hand, involve changes at the atomic nucleus. This is where protons and neutrons, collectively called nucleons, reside. Nuclear reactions can alter the number of protons in an atom's nucleus, thereby transmuting one element into another. This is fundamentally different from chemical reactions, where the identity of the elements remains constant. The process of nuclear fission, where a heavy nucleus splits into smaller nuclei, and nuclear fusion, where light nuclei combine to form a heavier nucleus, are prime examples of this transformative power.
Energy Transformations: A World Apart
The energy changes associated with these two reaction types are vastly different. Chemical reactions involve relatively small changes in energy, typically measured in kilojoules per mole (kJ/mol). These energy shifts result from the breaking and forming of chemical bonds. Burning wood, for instance, is a highly exothermic chemical reaction that releases energy in the form of heat and light.
Nuclear reactions, however, release or absorb far greater amounts of energy, typically measured in megajoules per mole (MJ/mol) or even gigajoules per mole (GJ/mol). This enormous energy difference stems from the immense forces holding the nucleus together—the strong nuclear force—which is far stronger than the electromagnetic forces responsible for chemical bonds. The energy released in a nuclear explosion dwarfs that of any chemical explosion of comparable mass. This massive energy difference is a defining characteristic and explains the immense power of nuclear weapons and the potential of nuclear fusion as a future energy source.
Particle Involvement: A Tale of Two Worlds
The particles involved in the reactions also differ significantly. Chemical reactions involve the interaction of electrons, which are relatively light particles orbiting the nucleus. These interactions determine the formation and breakage of chemical bonds. The electrons' participation creates ionic or covalent bonds that lead to molecular changes. The nucleus remains largely unaffected during chemical reactions.
Nuclear reactions, conversely, involve the direct interaction of protons and neutrons within the nucleus. These interactions can result in the emission of high-energy particles like alpha particles (helium nuclei), beta particles (electrons or positrons), and gamma rays (high-energy photons). The release of these particles is a hallmark of radioactive decay, a specific type of nuclear reaction. Neutrons also play a critical role in nuclear fission, initiating a chain reaction that leads to the release of enormous energy. In nuclear fusion, protons overcome electrostatic repulsion to merge, creating a larger nucleus and releasing enormous amounts of energy.
Reaction Rates: A Matter of Speed
The speed at which chemical and nuclear reactions occur also differs dramatically. Chemical reactions can range from extremely fast (explosions) to very slow (corrosion). The rate is influenced by factors such as temperature, pressure, concentration, and the presence of catalysts.
Nuclear reactions, particularly radioactive decay, proceed at a characteristic rate independent of external factors like temperature or pressure. This rate is governed by the half-life of the radioactive isotope, which is a constant that determines the time it takes for half of the nuclei in a sample to decay. While the rate of some nuclear reactions, like nuclear fission in a reactor, can be controlled, the underlying decay processes remain fundamentally independent of external factors. This predictability is crucial for applications like radioactive dating and nuclear medicine.
Types of Reactions: A Divergent Landscape
The diversity of chemical and nuclear reactions also showcases fundamental differences. Chemical reactions encompass a vast range, including:
- Synthesis reactions: Two or more substances combine to form a more complex substance.
- Decomposition reactions: A compound breaks down into simpler substances.
- Single displacement reactions: One element replaces another in a compound.
- Double displacement reactions: Two compounds exchange ions.
- Acid-base reactions: An acid reacts with a base to form salt and water.
- Redox reactions: Involve the transfer of electrons between chemical species.
Nuclear reactions, while less diverse in the overall sense, still exhibit several distinct categories:
- Radioactive decay: An unstable nucleus spontaneously emits particles or energy to become more stable. This includes alpha decay, beta decay, and gamma decay.
- Nuclear fission: A heavy nucleus splits into smaller nuclei, releasing a large amount of energy and more neutrons.
- Nuclear fusion: Light nuclei combine to form a heavier nucleus, releasing an even larger amount of energy.
Each type of nuclear reaction has specific characteristics regarding the energy released, the particles emitted, and the resulting isotopes produced. Understanding these distinctions is crucial for applications in nuclear power generation, nuclear medicine, and the study of stellar nucleosynthesis.
Real-World Applications: A Stark Contrast
The differences between chemical and nuclear reactions translate into vastly different applications. Chemical reactions are essential to all aspects of life and industry. They underpin processes such as digestion, photosynthesis, combustion, and the production of countless materials, from plastics to pharmaceuticals.
Nuclear reactions, due to their immense energy release, have primarily been used in:
- Nuclear power generation: Nuclear fission reactions provide a significant source of electricity in several countries.
- Nuclear weapons: The destructive power of nuclear fission and fusion underlies the development of nuclear weapons.
- Nuclear medicine: Radioactive isotopes are used for diagnostic imaging (PET scans) and therapeutic treatments (radiation therapy).
- Radioactive dating: The decay of radioactive isotopes is used to determine the age of ancient artifacts and geological formations.
The contrasting applications reflect the inherent differences in the energy scales and the underlying mechanisms of chemical and nuclear reactions.
Conclusion: A World of Differences, a Universe of Applications
Chemical and nuclear reactions represent two fundamentally different ways matter can transform. While chemical reactions involve the rearrangement of atoms within molecules, nuclear reactions involve changes within the atomic nucleus itself. This difference leads to significant disparities in energy changes, particle involvement, reaction rates, and real-world applications. Understanding these fundamental differences is essential for appreciating the complexity of the physical world and harnessing the power of both chemical and nuclear processes for the benefit of humankind. The vast energy potential of nuclear reactions, while powerful, necessitates careful and responsible management to mitigate potential risks. Conversely, the intricacies of chemical reactions continue to provide a vast playground for innovation across numerous fields. The continuing research and development in both areas promise exciting possibilities for the future.
Latest Posts
Latest Posts
-
Factors That Influence The Elasticity Of Supply
Mar 17, 2025
-
Describe How The Atoms In A Compound Are Held Together
Mar 17, 2025
-
How Is Absorbance Linked To Rate Of Reaction
Mar 17, 2025
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
-
Are Antiparalell Beta Sheets Mrore Stable
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Nuclear And Chemical Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
