Are Nonpolar Molecules Hydrophobic Or Hydrophilic
Muz Play
Mar 26, 2025 · 5 min read
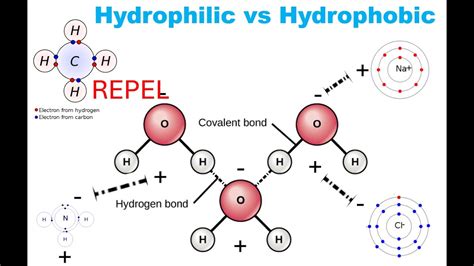
Table of Contents
Are Nonpolar Molecules Hydrophobic or Hydrophilic? Understanding Polarity and Molecular Interactions
The question of whether nonpolar molecules are hydrophobic or hydrophilic is fundamental to understanding chemistry and biology. The answer, simply put, is hydrophobic. However, a complete understanding requires delving into the nature of polarity, intermolecular forces, and the behavior of molecules in aqueous solutions. This article will explore these concepts in detail, providing a comprehensive explanation for both beginners and those seeking a deeper understanding.
Understanding Polarity: The Foundation of Hydrophobicity and Hydrophilicity
Polarity refers to the distribution of electrical charge within a molecule. A polar molecule has a positive and a negative end due to an uneven distribution of electrons. This occurs when one atom in the molecule is significantly more electronegative than the others, attracting electrons more strongly and creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atoms. Water (H₂O) is a classic example of a polar molecule. The oxygen atom is more electronegative than the hydrogen atoms, leading to a bent molecular geometry and a partial negative charge on the oxygen and partial positive charges on the hydrogens.
Nonpolar molecules, conversely, have an even distribution of electrical charge. This usually arises when the atoms within the molecule have similar electronegativities or when the molecular geometry cancels out any potential dipole moments. Examples include methane (CH₄) and carbon dioxide (CO₂).
Intermolecular Forces: The Driving Forces Behind Molecular Interactions
The behavior of molecules in solution, particularly their interaction with water, is governed by intermolecular forces. These are the attractive forces between molecules. Several types of intermolecular forces exist, including:
-
Hydrogen bonding: A strong type of dipole-dipole attraction involving a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom. This is crucial for the properties of water.
-
Dipole-dipole interactions: Attractions between the positive end of one polar molecule and the negative end of another.
-
London Dispersion Forces (LDFs): These are weak, temporary attractions between molecules caused by instantaneous fluctuations in electron distribution. Even nonpolar molecules experience LDFs.
Hydrophilic vs. Hydrophobic: A Tale of Two Interactions
Hydrophilic (water-loving) molecules readily interact with water. This is typically due to their ability to form hydrogen bonds or dipole-dipole interactions with water molecules. Polar molecules, including sugars and many amino acids, are hydrophilic. Their charged or partially charged regions can interact favorably with the charged regions of water molecules.
Hydrophobic (water-fearing) molecules, on the other hand, tend to avoid contact with water. This is not because they repel water, but rather because they cannot effectively participate in the strong hydrogen bonding network that water molecules form with each other. Nonpolar molecules, lacking significant charge separation, primarily interact through weak London Dispersion Forces. These forces are much weaker than the hydrogen bonding between water molecules. Therefore, the water molecules tend to cluster around themselves, forcing the hydrophobic molecules to aggregate together.
Why Nonpolar Molecules are Hydrophobic: A Detailed Explanation
The hydrophobicity of nonpolar molecules stems from the energetic favorability of water molecules interacting with each other rather than with nonpolar molecules. Water molecules are highly cohesive, forming a complex, interconnected network through hydrogen bonding. Introducing a nonpolar molecule disrupts this network. To accommodate the nonpolar molecule, water molecules would have to rearrange their hydrogen bonds, resulting in a less energetically favorable arrangement. Therefore, the system minimizes its energy by keeping the nonpolar molecules clustered together, minimizing the disruption of the water's hydrogen bonding network. This clustering effect is often referred to as the hydrophobic effect.
This effect is crucial for many biological processes. For example, the hydrophobic interactions between amino acid side chains are a major driving force in protein folding, ensuring that the hydrophobic regions are tucked away inside the protein's core, away from the aqueous environment.
Amphipathic Molecules: Bridging the Gap Between Hydrophilic and Hydrophobic
Some molecules possess both hydrophilic and hydrophobic regions. These are known as amphipathic molecules. A prime example is a phospholipid, a key component of cell membranes. Phospholipids have a hydrophilic head (polar phosphate group) and two hydrophobic tails (nonpolar fatty acid chains). In an aqueous environment, these molecules spontaneously self-assemble into structures like micelles or bilayers, with the hydrophilic heads facing the water and the hydrophobic tails clustered together, minimizing contact with water. This self-assembly is a direct consequence of the hydrophobic effect.
Practical Applications and Implications
The understanding of hydrophobicity and hydrophilicity is crucial in numerous fields:
-
Pharmaceutical science: Drug design often involves considering the hydrophobicity/hydrophilicity of drug molecules to ensure their solubility, absorption, and distribution within the body.
-
Materials science: The design of materials with specific wetting properties, such as waterproof fabrics or self-cleaning surfaces, relies on manipulating the hydrophobicity or hydrophilicity of their constituent molecules.
-
Environmental science: Understanding how pollutants interact with water is crucial for remediation efforts. Hydrophobic pollutants, for example, tend to accumulate in sediments and soils.
-
Biotechnology: Techniques like chromatography separate molecules based on their hydrophobicity.
Conclusion: A Holistic View of Molecular Interactions
In conclusion, while the answer to the question – are nonpolar molecules hydrophobic or hydrophilic? – is definitively hydrophobic, the underlying principles are far more intricate. The hydrophobicity of nonpolar molecules is a consequence of the energetic favorability of water molecules interacting with each other through strong hydrogen bonds, leading to the clustering of nonpolar molecules to minimize disruption of this network. This phenomenon, the hydrophobic effect, is a fundamental force shaping the structures and functions of biological systems and influencing a wide range of applications across various scientific disciplines. Understanding this interplay between polarity, intermolecular forces, and the hydrophobic effect is key to grasping the complexity and beauty of the molecular world.
Latest Posts
Latest Posts
-
What Is Stronger C C Bond Or C Cl Bond
Mar 29, 2025
-
Where Is The Bacterial Chromosome Located
Mar 29, 2025
-
Write The Chemical Formula For This Molecule
Mar 29, 2025
-
How To Calculate Velocity From Flow Rate
Mar 29, 2025
-
Write The Iupac Names Of The Given Carboxylic Acids
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Are Nonpolar Molecules Hydrophobic Or Hydrophilic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
