Arrange The Compounds By Their Solubility In Water
Muz Play
Apr 01, 2025 · 5 min read
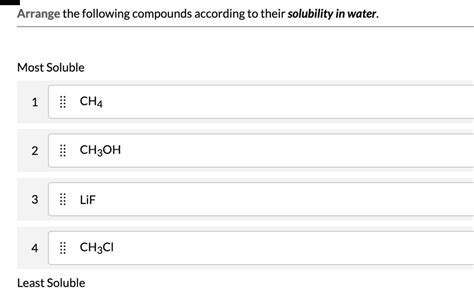
Table of Contents
Arranging Compounds by Water Solubility: A Comprehensive Guide
Understanding the solubility of compounds in water is crucial in various fields, from chemistry and pharmaceuticals to environmental science and geology. The ability of a substance to dissolve in water depends on several factors, primarily the interplay between the solute's and solvent's intermolecular forces. This article provides a comprehensive guide to arranging compounds based on their solubility in water, covering the key principles, factors influencing solubility, and practical examples.
Understanding Water Solubility
Water, a highly polar solvent, dissolves substances based on the principle "like dissolves like." This means polar and ionic compounds tend to dissolve readily in water, while nonpolar compounds are generally insoluble. The dissolution process involves the breaking of solute-solute interactions and solvent-solvent interactions, followed by the formation of solute-solvent interactions. If the energy released during the formation of solute-solvent interactions is greater than the energy required to break the initial interactions, the compound is soluble. Otherwise, it remains insoluble.
Factors Affecting Water Solubility
Several factors significantly influence the solubility of a compound in water:
-
Polarity: The most crucial factor. Polar compounds with dipole moments possess partial positive and negative charges, allowing them to interact favorably with water molecules through dipole-dipole interactions and hydrogen bonding. Ionic compounds, with full charges on their ions, exhibit even stronger interactions with water, leading to high solubility.
-
Hydrogen Bonding: The presence of hydrogen atoms bonded to highly electronegative atoms (like oxygen, nitrogen, or fluorine) allows for strong hydrogen bonds with water molecules, significantly enhancing solubility. Alcohols, carboxylic acids, and amines are good examples of compounds exhibiting high solubility due to hydrogen bonding.
-
Molecular Weight: As molecular weight increases, solubility generally decreases, especially for nonpolar compounds. Larger molecules have stronger London Dispersion Forces (LDFs) within themselves, making it energetically less favorable to break these interactions and interact with water molecules.
-
Temperature: The effect of temperature on solubility varies depending on the compound. Generally, the solubility of solids in water increases with temperature, as the increased kinetic energy facilitates the breaking of solute-solvent interactions. However, the solubility of gases in water typically decreases with increasing temperature.
-
Pressure: Pressure has a minimal effect on the solubility of solids and liquids in water, but it significantly influences the solubility of gases. According to Henry's Law, the solubility of a gas in water is directly proportional to the partial pressure of the gas above the solution.
Classifying Compounds Based on Solubility
Compounds can be broadly categorized into several solubility classes based on their solubility in water:
-
Very Soluble: Compounds that dissolve readily in water, typically dissolving greater than 1 gram per 100 mL of water at room temperature. Examples include sodium chloride (NaCl), glucose, and many small alcohols.
-
Soluble: Compounds that dissolve moderately well in water, generally dissolving between 1 gram and 0.1 gram per 100 mL of water at room temperature. Examples include many salts of alkali metals and some small organic molecules.
-
Slightly Soluble: Compounds that have limited solubility in water, dissolving between 0.1 gram and 0.01 gram per 100 mL of water at room temperature. Examples include some metal hydroxides and sparingly soluble salts.
-
Insoluble (or Practically Insoluble): Compounds that exhibit very low solubility in water, dissolving less than 0.01 gram per 100 mL of water at room temperature. Many nonpolar organic compounds like hydrocarbons fall under this category.
Note: These are general guidelines; the exact solubility values can vary depending on the specific compound and experimental conditions.
Practical Examples and Arranging Compounds
Let's arrange a set of compounds based on their predicted solubility in water:
Compounds:
- Sodium chloride (NaCl)
- Hexane (C₆H₁₄)
- Ethanol (CH₃CH₂OH)
- Benzene (C₆H₆)
- Sucrose (C₁₂H₂₂O₁₁)
- Calcium sulfate (CaSO₄)
- Octanol (CH₃(CH₂)₇OH)
- Acetic acid (CH₃COOH)
Arranging by Decreasing Solubility:
-
Sodium chloride (NaCl): A highly soluble ionic compound due to strong ion-dipole interactions with water.
-
Sucrose (C₁₂H₂₂O₁₁): A very soluble polar compound; the numerous hydroxyl groups allow extensive hydrogen bonding with water.
-
Ethanol (CH₃CH₂OH): A soluble polar compound, exhibiting hydrogen bonding with water.
-
Acetic acid (CH₃COOH): A soluble polar compound, forming hydrogen bonds with water molecules through both the hydroxyl and carbonyl groups.
-
Calcium sulfate (CaSO₄): A slightly soluble ionic compound. While ionic, its lattice energy is relatively high, making it less soluble compared to NaCl.
-
Octanol (CH₃(CH₂)₇OH): Slightly soluble. While it has a hydroxyl group capable of hydrogen bonding, the long nonpolar hydrocarbon chain dominates, reducing its overall solubility.
-
Benzene (C₆H₆): Practically insoluble. A nonpolar compound with only weak London Dispersion Forces; it does not interact favorably with water.
-
Hexane (C₆H₁₄): Practically insoluble. A nonpolar hydrocarbon with very low solubility in water.
Advanced Considerations and Applications
The principles discussed above form the basis for predicting and understanding water solubility. However, more complex scenarios may require considering additional factors:
-
pH: The pH of the solution significantly affects the solubility of acidic or basic compounds. Changing the pH can convert a poorly soluble compound into a more soluble form (e.g., dissolving a slightly soluble metal hydroxide in an acidic solution).
-
Complexation: The formation of metal complexes can enhance the solubility of otherwise insoluble metal salts. Ligands that bind to the metal ion can make the complex more polar and increase its interaction with water.
-
Salting-out effect: Adding a salt to a solution can decrease the solubility of nonpolar substances. The salt ions compete with the nonpolar solute for interaction with water molecules, effectively "salting out" the nonpolar compound.
-
Micelles and Surfactants: Surfactants, amphiphilic molecules with both polar and nonpolar parts, can form micelles in water. These micelles can encapsulate nonpolar compounds, increasing their apparent solubility.
The ability to arrange compounds based on water solubility finds widespread application in:
-
Pharmaceutical sciences: Solubility determines the bioavailability of drugs. Designing drugs with appropriate solubility is crucial for effective delivery and absorption.
-
Environmental chemistry: Understanding solubility is critical in assessing the fate and transport of pollutants in aquatic environments.
-
Geochemistry: Solubility dictates the presence and distribution of minerals in geological systems.
This article provides a framework for understanding and predicting water solubility. While generalizations are helpful, remember that the precise solubility of a given compound depends on many interacting factors and should be determined through experimentation or advanced computational techniques whenever high accuracy is required. The provided examples highlight the fundamental principles, aiding in the classification and arrangement of compounds based on their interaction with water.
Latest Posts
Latest Posts
-
How Are Ionic And Covalent Bonding Similar
Apr 02, 2025
-
What Is The Electron Configuration Of Neon
Apr 02, 2025
-
In Which Phase Of Cellular Respiration Is Water Made
Apr 02, 2025
-
How To Measure Current On A Breadboard
Apr 02, 2025
-
Chapter 1 Lab Investigation The Language Of Anatomy
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Arrange The Compounds By Their Solubility In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
