Atoms Of The Same Element That Have Different Masses
Muz Play
Mar 27, 2025 · 6 min read
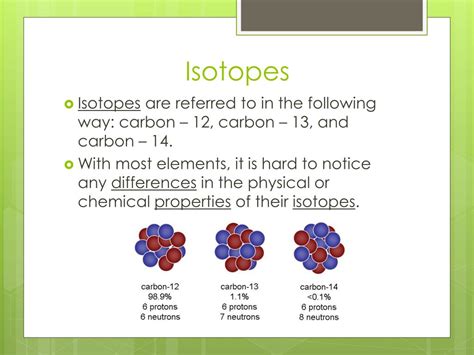
Table of Contents
- Atoms Of The Same Element That Have Different Masses
- Table of Contents
- Atoms of the Same Element That Have Different Masses: Isotopes Explained
- What are Isotopes?
- Notation of Isotopes
- Properties of Isotopes
- Types of Isotopes: Stable vs. Radioactive
- 1. Stable Isotopes:
- 2. Radioactive Isotopes (Radioisotopes):
- Applications of Isotopes
- 1. Nuclear Medicine:
- 2. Radiocarbon Dating:
- 3. Geological Dating:
- 4. Industrial Applications:
- 5. Environmental Studies:
- 6. Scientific Research:
- Isotopic Fractionation:
- Isotopic Abundance:
- Conclusion:
- Latest Posts
- Latest Posts
- Related Post
Atoms of the Same Element That Have Different Masses: Isotopes Explained
Atoms, the fundamental building blocks of matter, are often depicted as simple spheres. However, the reality is far more nuanced. While atoms of the same element share the same number of protons (defining their atomic number and element identity), they can possess differing numbers of neutrons. This leads to the existence of isotopes, atoms of the same element with different masses. Understanding isotopes is crucial to various fields, from nuclear medicine to geological dating and beyond. This comprehensive article delves into the intricacies of isotopes, exploring their properties, applications, and significance in the scientific world.
What are Isotopes?
Isotopes are variations of a chemical element that differ in neutron number, although they have the same number of protons and electrons. This means they have the same atomic number (Z) but different mass numbers (A). The mass number (A) represents the total number of protons and neutrons in the atom's nucleus. Since neutrons contribute to the mass but not the charge, isotopes of the same element exhibit nearly identical chemical properties but differ slightly in their physical properties, primarily mass.
For example, consider carbon (C), with an atomic number of 6. The most common isotope is carbon-12 (¹²C), containing 6 protons and 6 neutrons. However, carbon also exists as carbon-13 (¹³C) with 6 protons and 7 neutrons, and carbon-14 (¹⁴C) with 6 protons and 8 neutrons. All three are isotopes of carbon, but their masses differ due to the varying neutron counts.
Notation of Isotopes
Isotopes are commonly represented using a specific notation:
- ¹²C: The superscript (12) represents the mass number (A), the sum of protons and neutrons.
- C: The symbol represents the element (Carbon).
- 6: The subscript (6 – often omitted) represents the atomic number (Z), the number of protons.
This notation clearly identifies the specific isotope being discussed.
Properties of Isotopes
While isotopes of the same element exhibit nearly identical chemical behavior, their physical properties, particularly mass, differ. This difference in mass influences several physical characteristics:
- Mass: The most obvious difference lies in their mass. Heavier isotopes have a larger mass due to the increased number of neutrons.
- Density: Isotopic differences in mass can lead to slight variations in density.
- Nuclear Stability: Not all isotopes are stable. Some are radioactive, meaning their nuclei spontaneously decay, emitting radiation as they transform into a different, more stable isotope or element. The stability of an isotope depends on the neutron-to-proton ratio in its nucleus.
- Rate of Diffusion: Heavier isotopes diffuse slower than lighter ones due to their greater mass. This is exploited in techniques like isotopic fractionation.
Types of Isotopes: Stable vs. Radioactive
Isotopes are categorized into two primary types based on their nuclear stability:
1. Stable Isotopes:
These isotopes possess a stable nucleus and do not undergo radioactive decay. They remain unchanged over time. Many elements have one or more stable isotopes. For example, carbon has two stable isotopes, ¹²C and ¹³C.
2. Radioactive Isotopes (Radioisotopes):
Radioactive isotopes possess unstable nuclei and undergo radioactive decay, emitting various types of radiation (alpha, beta, gamma) as they transition to a more stable state. This decay process follows a predictable pattern, characterized by a half-life – the time it takes for half of the radioactive atoms in a sample to decay. Radioisotopes find extensive applications in various fields, discussed later. Examples include carbon-14 (¹⁴C), used in radiocarbon dating, and iodine-131 (¹³¹I), used in medical treatments.
Applications of Isotopes
The distinct properties of isotopes, particularly their differing masses and radioactive decay characteristics, make them invaluable tools in numerous fields:
1. Nuclear Medicine:
Radioactive isotopes are extensively used in medical diagnostics and therapy. For example:
- Diagnostic Imaging: Radioisotopes like technetium-99m (⁹⁹mTc) are used in various imaging techniques, such as single-photon emission computed tomography (SPECT) and bone scans, to visualize internal organs and detect abnormalities.
- Radiotherapy: Radioactive isotopes like iodine-131 (¹³¹I) and cobalt-60 (⁶⁰Co) are employed in radiotherapy to target and destroy cancerous cells.
2. Radiocarbon Dating:
Carbon-14 (¹⁴C), a radioactive isotope of carbon, is crucial in radiocarbon dating. This technique determines the age of organic materials up to approximately 50,000 years old. The decay rate of ¹⁴C is constant and predictable, allowing scientists to estimate the time elapsed since the organism died.
3. Geological Dating:
Various radioactive isotopes, such as uranium-238 (²³⁸U), uranium-235 (²³⁵U), and potassium-40 (⁴⁰K), are used to determine the age of rocks and minerals. By analyzing the decay products of these isotopes, geologists can establish the geological timescale and understand Earth's history.
4. Industrial Applications:
Isotopes find diverse applications in various industries:
- Tracer Studies: Isotopes are used as tracers to track the movement of materials in industrial processes, such as pipelines and chemical reactions.
- Thickness Gauges: Radioactive isotopes are used in instruments to measure the thickness of materials like paper, plastic film, and metal sheets.
- Sterilization: Radioactive isotopes are used to sterilize medical equipment and food products.
5. Environmental Studies:
Isotopes are valuable tools in environmental science:
- Water Tracing: Isotopes of hydrogen and oxygen are used to trace the movement of water in groundwater systems and hydrological cycles.
- Pollution Studies: Isotopic techniques help identify the sources and pathways of pollutants in the environment.
6. Scientific Research:
Isotopes play a critical role in various scientific research areas:
- Nuclear Physics: Studying the properties and behavior of isotopes contributes to our understanding of nuclear structure and forces.
- Chemistry: Isotope effects are studied to understand reaction mechanisms and molecular dynamics.
- Biology: Isotopic labeling is used to study metabolic pathways and biological processes.
Isotopic Fractionation:
Isotopic fractionation refers to the process where isotopes of the same element become separated during physical, chemical, or biological processes. This separation occurs because the slightly different masses of isotopes affect their reaction rates and physical properties. For example, lighter isotopes tend to evaporate more readily than heavier isotopes. This process is crucial in understanding various natural phenomena, such as the isotopic composition of water and the variations in carbon isotopes in plants.
Isotopic Abundance:
Isotopic abundance refers to the relative proportions of different isotopes of an element found in a natural sample. The abundance of each isotope varies depending on the element and its origin. These abundances are often expressed as percentages and are essential data in various fields, including geochemistry and environmental science. For example, the isotopic abundance of carbon-12 is about 98.9%, while carbon-13's abundance is about 1.1%.
Conclusion:
Isotopes, atoms of the same element with differing masses, are far from mere scientific curiosities. Their unique properties and applications are indispensable across a vast range of disciplines, from medicine and geology to environmental science and industrial processes. Understanding the fundamental nature of isotopes, their stability, and their various applications is crucial for advancing scientific knowledge and addressing real-world challenges. The ongoing research into isotopes continues to reveal new insights into the workings of the universe and expands the potential for their use in improving human life. From diagnosing diseases to dating ancient artifacts and understanding environmental changes, the significance of isotopes remains undeniable and continually evolving.
Latest Posts
Latest Posts
-
Packing Efficiency Of Body Centered Cubic
Mar 31, 2025
-
What Is Reversible And Irreversible Process In Thermodynamics
Mar 31, 2025
-
Moment Of Inertia Of Uniform Rod
Mar 31, 2025
-
Keratin And Collagen Are Examples Of Which Class Of Proteins
Mar 31, 2025
-
Job Order Costing Vs Process Costing
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Atoms Of The Same Element That Have Different Masses . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
