Ca Oh 2 Strong Or Weak Base
Muz Play
Mar 18, 2025 · 6 min read
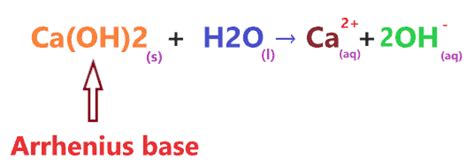
Table of Contents
Ca(OH)₂: Strong Base or Weak Base? Understanding its Properties and Reactions
Determining whether calcium hydroxide, Ca(OH)₂, is a strong or weak base is crucial for understanding its behavior in chemical reactions and its applications in various fields. This comprehensive article will delve into the properties of Ca(OH)₂, clarifying its classification as a strong base and exploring its chemical behavior, practical applications, and safety considerations.
Understanding the Concept of Strong and Weak Bases
Before classifying Ca(OH)₂, let's establish a clear understanding of the difference between strong and weak bases. A strong base is a base that completely dissociates into its constituent ions (cations and anions) when dissolved in water. This means that essentially all of the hydroxide ions (OH⁻) are released into the solution. Conversely, a weak base only partially dissociates, meaning only a small fraction of the base molecules release hydroxide ions. The extent of dissociation is quantified by the base dissociation constant (Kb). Strong bases have very large Kb values, while weak bases have small Kb values.
Why Ca(OH)₂ is Classified as a Strong Base
Calcium hydroxide, commonly known as slaked lime or hydrated lime, is a strong base because it completely dissociates in water. The dissociation reaction can be represented as follows:
Ca(OH)₂(s) → Ca²⁺(aq) + 2OH⁻(aq)
This equation shows that one mole of solid Ca(OH)₂ completely breaks down into one mole of calcium ions (Ca²⁺) and two moles of hydroxide ions (OH⁻) when dissolved in water. This complete dissociation is the defining characteristic of a strong base. The high concentration of hydroxide ions generated contributes to the strong alkaline nature of Ca(OH)₂ solutions.
Evidence Supporting its Strong Base Nature
Several pieces of evidence reinforce Ca(OH)₂'s classification as a strong base:
-
High pH values: Aqueous solutions of Ca(OH)₂ exhibit very high pH values, typically above 12, indicating a high concentration of hydroxide ions. The pH scale measures the concentration of hydrogen ions (H⁺), but since pH + pOH = 14, a high pH directly translates to a high pOH and thus a high hydroxide ion concentration.
-
Complete dissociation in dilute solutions: While the solubility of Ca(OH)₂ is relatively low compared to other strong bases like NaOH or KOH, the portion that does dissolve dissociates completely. This complete dissociation is the key factor in classifying it as a strong base. The limited solubility doesn't negate its strong base nature; it simply means that the concentration of hydroxide ions in a saturated solution is less than for highly soluble strong bases.
-
Reaction with acids: Ca(OH)₂ readily reacts with acids in a neutralization reaction, producing water and a salt. This reaction proceeds virtually to completion, further demonstrating the complete availability of hydroxide ions for reaction. For instance, the reaction with hydrochloric acid (HCl) is:
Ca(OH)₂(aq) + 2HCl(aq) → CaCl₂(aq) + 2H₂O(l)
This complete neutralization reaction is characteristic of reactions involving strong bases.
Practical Applications of Ca(OH)₂
The strong base properties of Ca(OH)₂ contribute to its wide range of applications across various industries:
1. Construction and Building Materials:
-
Mortar and Plaster: Ca(OH)₂ is a crucial component of mortar and plaster, providing strength and binding properties. The reaction of Ca(OH)₂ with carbon dioxide in the air forms calcium carbonate (CaCO₃), contributing to the hardening of these materials. This process, known as carbonation, is a slow reaction and is an example of its base properties in action.
-
Lime Stabilization of Soil: Ca(OH)₂ improves soil stability and reduces permeability, making it useful in soil stabilization projects for road construction and other civil engineering applications. The alkaline nature of Ca(OH)₂ alters the soil's chemical properties.
2. Wastewater Treatment:
-
pH Adjustment: Ca(OH)₂ is used to adjust the pH of wastewater, neutralizing acidic components and improving the efficiency of wastewater treatment processes. The addition of Ca(OH)₂ raises the pH, bringing it to an optimal range for biological processes.
-
Phosphate Removal: In wastewater treatment plants, Ca(OH)₂ helps to remove phosphates, which are significant pollutants contributing to eutrophication in water bodies. The reaction forms insoluble calcium phosphate, facilitating its removal.
3. Chemical Industry:
-
Production of other chemicals: Ca(OH)₂ serves as a raw material in the production of several chemicals, including calcium hypochlorite (Ca(ClO)₂), a common bleaching agent.
-
Neutralization of acidic spills: Its strong base properties make Ca(OH)₂ useful in neutralizing accidental spills of acids, minimizing environmental damage. This application relies on its rapid and efficient neutralization of acidic substances.
4. Food Industry:
- Food additive: In some cases, Ca(OH)₂ is used as a food additive (E526), primarily for adjusting pH and processing various foods. Its usage is heavily regulated to ensure safety.
5. Agriculture:
- Soil amendment: Ca(OH)₂ can be used to amend acidic soils, increasing their pH and improving the availability of nutrients for plant growth. It modifies the soil's chemical environment to create more favorable conditions for plant life.
Safety Precautions when Handling Ca(OH)₂
Ca(OH)₂, despite its widespread applications, is a corrosive substance and requires careful handling. Direct contact with skin or eyes can cause severe irritation or burns. Inhalation of Ca(OH)₂ dust can irritate the respiratory system. Therefore, appropriate personal protective equipment (PPE), including gloves, eye protection, and respirators, should be used when handling Ca(OH)₂. Proper ventilation is also essential to minimize dust inhalation risks. In case of accidental contact or ingestion, immediate medical attention should be sought.
Differentiating Ca(OH)₂ from Weak Bases
It's important to highlight the distinction between Ca(OH)₂ and weak bases. Weak bases, such as ammonia (NH₃) or pyridine (C₅H₅N), only partially dissociate in water, resulting in a lower concentration of hydroxide ions compared to a strong base at the same concentration. This difference significantly impacts their reactivity and applications. Weak bases do not completely neutralize acids, and their reactions are equilibrium-driven, meaning the extent of the reaction is limited.
Conclusion: Understanding the Importance of Ca(OH)₂'s Strong Base Nature
The complete dissociation of Ca(OH)₂ in water establishes it firmly as a strong base. This fundamental property underlies its wide range of applications in construction, wastewater treatment, chemical manufacturing, food processing, and agriculture. Understanding its behavior as a strong base is critical for safe handling and effective utilization in various industrial processes. While its solubility limits the maximum hydroxide ion concentration achievable, its complete dissociation in solution makes it a powerful and versatile chemical compound. Always remember to prioritize safety when working with Ca(OH)₂, ensuring appropriate precautions are taken to prevent any potential hazards. Further research into its properties continues to reveal new and exciting applications for this important strong base.
Latest Posts
Latest Posts
-
Is S More Electronegative Than O
Mar 18, 2025
-
How Many Valence Electrons Are In Sulfur
Mar 18, 2025
-
What Is The Difference Between Volume Measurements And Capacities
Mar 18, 2025
-
In A Polar Covalent Bond Electrons Are Shared
Mar 18, 2025
-
Why Does Standard Deviation Decrease With Sample Size
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Ca Oh 2 Strong Or Weak Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
