Chemical Potential And Gibbs Free Energy
Muz Play
Mar 20, 2025 · 6 min read
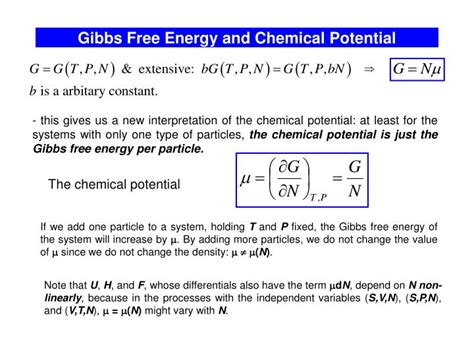
Table of Contents
Chemical Potential and Gibbs Free Energy: A Deep Dive
Understanding chemical potential and Gibbs free energy is crucial for grasping the spontaneity and equilibrium of chemical and physical processes. These thermodynamic concepts are fundamental to numerous fields, from chemistry and materials science to biochemistry and environmental engineering. This article provides a comprehensive exploration of both, emphasizing their relationship and practical applications.
What is Chemical Potential?
Chemical potential, denoted by μ (mu), is a thermodynamic quantity that measures the change in Gibbs free energy of a system when a single particle (atom, molecule, or ion) is added to the system while keeping the temperature, pressure, and number of other particles constant. In simpler terms, it represents the "escaping tendency" of a component from a phase or system. A higher chemical potential signifies a greater tendency to escape.
Understanding the Definition
Think of it like this: if you have a container of gas under pressure, the gas molecules have a high chemical potential. They're constantly bumping into each other and the walls, eager to escape. Conversely, a molecule in a dilute solution has a lower chemical potential; it's less likely to escape because it's surrounded by a lot of solvent.
The chemical potential is a partial molar Gibbs free energy. Mathematically, it's defined as:
μᵢ = (∂G/∂nᵢ)<sub>T,P,nⱼ</sub>
Where:
- μᵢ is the chemical potential of component i
- G is the Gibbs free energy
- nᵢ is the number of moles of component i
- T is the temperature
- P is the pressure
- nⱼ represents the number of moles of all other components, held constant.
This equation states that the chemical potential of a component is the rate of change of the Gibbs free energy with respect to the change in the amount of that component, while all other factors are kept constant.
Factors Affecting Chemical Potential
Several factors influence the chemical potential of a substance:
- Temperature: Increasing temperature generally increases the chemical potential, as molecules have more kinetic energy and are more likely to escape.
- Pressure: Increasing pressure usually increases the chemical potential, particularly for gases. Higher pressure forces molecules closer together, increasing their "escaping tendency".
- Concentration: For solutions, higher concentration leads to a higher chemical potential. The more solute molecules present, the more likely they are to escape.
- Intermolecular Forces: Strong intermolecular forces (like hydrogen bonding) lower the chemical potential. Molecules are more strongly bound to each other and less likely to escape.
Chemical Potential and Equilibrium
At equilibrium, the chemical potential of a component is the same in all phases or systems where that component exists. This is a fundamental principle in phase equilibria and chemical reactions. For example, consider a liquid in equilibrium with its vapor. The chemical potential of the liquid phase equals the chemical potential of the vapor phase. If the chemical potentials are different, net transfer of the substance will occur until equilibrium is reached.
What is Gibbs Free Energy?
Gibbs free energy (G), named after Josiah Willard Gibbs, is a thermodynamic potential that measures the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. It's a crucial concept for predicting the spontaneity of a process.
Understanding Spontaneity
A process is spontaneous under constant temperature and pressure conditions if its Gibbs free energy change (ΔG) is negative. If ΔG is positive, the process is non-spontaneous under these conditions, and requires external energy input to occur. If ΔG is zero, the system is at equilibrium.
The Gibbs Free Energy Equation
The Gibbs free energy is defined by the equation:
G = H - TS
Where:
- G is the Gibbs free energy
- H is the enthalpy (heat content)
- T is the absolute temperature
- S is the entropy (disorder)
This equation shows that Gibbs free energy is a balance between enthalpy and entropy. A negative enthalpy change (exothermic reaction) favours spontaneity, while a positive entropy change (increase in disorder) also favours spontaneity. The temperature plays a crucial role in determining which factor dominates.
Gibbs Free Energy and Chemical Reactions
For chemical reactions, the change in Gibbs free energy (ΔG) is given by:
ΔG = ΔH - TΔS
This equation helps predict whether a reaction will proceed spontaneously at a given temperature. For example:
- ΔG < 0: The reaction is spontaneous under standard conditions.
- ΔG > 0: The reaction is non-spontaneous under standard conditions; energy must be supplied to make it proceed.
- ΔG = 0: The reaction is at equilibrium; there is no net change in the concentrations of reactants and products.
Standard Gibbs Free Energy Change
The standard Gibbs free energy change (ΔG°) represents the change in Gibbs free energy under standard conditions (usually 298 K and 1 atm pressure). It's a valuable tool for comparing the relative spontaneity of different reactions.
The Relationship Between Chemical Potential and Gibbs Free Energy
The chemical potential and Gibbs free energy are intimately linked. As mentioned earlier, the chemical potential of a component is the partial molar Gibbs free energy. This means that the chemical potential directly reflects the contribution of a component to the overall Gibbs free energy of the system.
Consider a system containing multiple components. The total Gibbs free energy is a function of temperature, pressure, and the number of moles of each component:
G = G(T, P, n₁, n₂, ... nᵢ)
The total differential of G is given by:
dG = (∂G/∂T)<sub>P,nᵢ</sub>dT + (∂G/∂P)<sub>T,nᵢ</sub>dP + Σᵢ (∂G/∂nᵢ)<sub>T,P,nⱼ</sub>dnᵢ
Using the definitions of chemical potential and other thermodynamic properties, we can rewrite this equation as:
dG = -SdT + VdP + Σᵢ μᵢdnᵢ
This equation is fundamental to understanding many thermodynamic processes. It highlights how changes in temperature, pressure, and the amount of each component affect the Gibbs free energy. The chemical potential terms directly contribute to the overall change in Gibbs free energy.
Applications of Chemical Potential and Gibbs Free Energy
The concepts of chemical potential and Gibbs free energy find widespread applications in diverse fields:
1. Phase Equilibria
Understanding phase transitions (like melting, boiling, and sublimation) requires knowledge of chemical potential. At equilibrium, the chemical potential of a substance is equal in all phases present. This principle is used to construct phase diagrams, which depict the conditions of temperature and pressure at which different phases coexist.
2. Chemical Reactions
Gibbs free energy is essential for predicting the spontaneity and equilibrium of chemical reactions. ΔG determines whether a reaction will proceed spontaneously under given conditions and also allows calculation of the equilibrium constant.
3. Electrochemistry
Chemical potential plays a critical role in electrochemistry. The difference in chemical potential between two electrodes drives the flow of electrons in a galvanic cell (battery).
4. Materials Science
Gibbs free energy is used to understand and predict the stability and transformations of different materials. For example, it helps determine the equilibrium phases in alloys and the conditions for the formation of new materials.
5. Biochemistry
In biochemistry, Gibbs free energy is crucial for understanding metabolic pathways and the spontaneity of biological reactions. The free energy change helps determine the feasibility of enzymatic reactions and other biochemical processes.
6. Environmental Science
Chemical potential is used to analyze the distribution of pollutants in the environment. Understanding the chemical potential of contaminants in different media helps predict their movement and fate.
Conclusion
Chemical potential and Gibbs free energy are cornerstones of thermodynamics, providing powerful tools for understanding and predicting the behavior of chemical and physical systems. Their interconnectedness is fundamental to numerous scientific and engineering disciplines. Mastering these concepts is essential for anyone seeking a deep understanding of the principles governing spontaneity, equilibrium, and transformations in various systems. From predicting the outcome of a chemical reaction to understanding phase transitions and designing new materials, these thermodynamic functions play a critical and versatile role.
Latest Posts
Latest Posts
-
When Bonds Are Broken Energy Is
Mar 21, 2025
-
What Does Amu Stand For In Chemistry
Mar 21, 2025
-
What Is The Si Unit For Weight
Mar 21, 2025
-
Is Water Boiling A Physical Change
Mar 21, 2025
-
What Is A Point Charge In Physics
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Chemical Potential And Gibbs Free Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
