Difference Between Bronsted Lowry And Lewis
Muz Play
Mar 20, 2025 · 6 min read
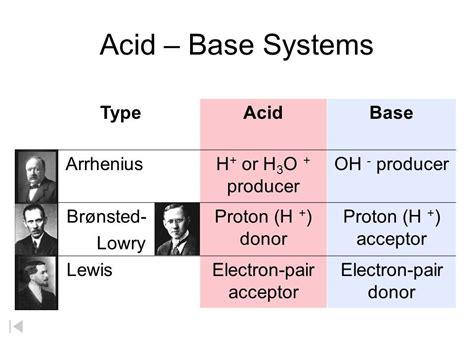
Table of Contents
Delving Deep into the Differences: Brønsted-Lowry vs. Lewis Acids and Bases
The world of chemistry often presents seemingly simple concepts that, upon closer inspection, reveal intricate layers of complexity. Acid-base chemistry is a prime example. While the Brønsted-Lowry and Lewis definitions of acids and bases are both widely used, they differ significantly in their scope and approach. Understanding these differences is crucial for a comprehensive grasp of chemical reactivity. This article aims to provide a detailed exploration of the distinctions between Brønsted-Lowry and Lewis acids and bases, clarifying their definitions, providing illustrative examples, and highlighting their applications.
Brønsted-Lowry Acids and Bases: A Proton-Centric Definition
The Brønsted-Lowry theory, proposed independently by Johannes Nicolaus Brønsted and Thomas Martin Lowry in 1923, centers on the transfer of protons (H⁺ ions). This theory expands upon the simpler Arrhenius definition, which limited acids to substances that produce H⁺ ions in aqueous solutions and bases to those that produce OH⁻ ions.
Defining Brønsted-Lowry Acids:
A Brønsted-Lowry acid is defined as a substance that donates a proton (H⁺) to another substance. This donation process is crucial; the acid doesn't simply contain protons; it must actively give them away. This ability to donate a proton is directly related to the acid's strength. Strong acids readily donate protons, while weak acids donate them less readily.
Examples of Brønsted-Lowry Acids:
- Hydrochloric acid (HCl): HCl readily donates a proton to water, forming H₃O⁺ (hydronium ion) and Cl⁻.
- Acetic acid (CH₃COOH): Acetic acid is a weak acid, donating a proton less readily than HCl. It establishes an equilibrium in water.
- Ammonium ion (NH₄⁺): Even a positively charged ion can act as a Brønsted-Lowry acid by donating a proton.
Defining Brønsted-Lowry Bases:
A Brønsted-Lowry base is defined as a substance that accepts a proton (H⁺) from another substance. The crucial aspect here is the base's ability to receive a proton, leading to the formation of a new bond between the proton and the base.
Examples of Brønsted-Lowry Bases:
- Water (H₂O): Water acts as a base when it accepts a proton from an acid like HCl.
- Ammonia (NH₃): Ammonia readily accepts a proton, forming the ammonium ion (NH₄⁺).
- Hydroxide ion (OH⁻): The hydroxide ion is a strong base, readily accepting a proton.
Conjugate Acid-Base Pairs:
A critical concept within the Brønsted-Lowry theory is the concept of conjugate acid-base pairs. When an acid donates a proton, the remaining species is called its conjugate base. Similarly, when a base accepts a proton, the resulting species is called its conjugate acid. These pairs are related by the difference of a single proton.
Example:
In the reaction between HCl and H₂O:
HCl (acid) + H₂O (base) ⇌ H₃O⁺ (conjugate acid) + Cl⁻ (conjugate base)
HCl and Cl⁻ form a conjugate acid-base pair, and H₂O and H₃O⁺ form another conjugate acid-base pair.
Lewis Acids and Bases: An Electron-Pair Perspective
The Lewis theory, introduced by Gilbert N. Lewis in 1923, provides a broader definition of acids and bases, focusing on the donation and acceptance of electron pairs. This theory transcends the limitations of the Brønsted-Lowry theory, encompassing reactions that don't involve proton transfer.
Defining Lewis Acids:
A Lewis acid is defined as a substance that accepts an electron pair. This acceptance forms a coordinate covalent bond, where both electrons in the bond originate from the same atom (the Lewis base). Lewis acids are often electron-deficient species, possessing vacant orbitals that can accommodate an electron pair.
Examples of Lewis Acids:
- Boron trifluoride (BF₃): BF₃ has an incomplete octet, readily accepting an electron pair from a Lewis base.
- Aluminum chloride (AlCl₃): Similar to BF₃, AlCl₃ acts as a Lewis acid due to its electron deficiency.
- Transition metal ions: Many transition metal ions, with their partially filled d-orbitals, can act as Lewis acids.
Defining Lewis Bases:
A Lewis base is defined as a substance that donates an electron pair. This donation forms a coordinate covalent bond with a Lewis acid. Lewis bases are typically species with lone pairs of electrons that can be shared.
Examples of Lewis Bases:
- Ammonia (NH₃): Ammonia possesses a lone pair of electrons that it can donate.
- Water (H₂O): Water also has lone pairs available for donation.
- Chloride ion (Cl⁻): The chloride ion has a lone pair and can act as a Lewis base.
The Broader Scope of Lewis Theory:
The Lewis theory encompasses a wider range of reactions than the Brønsted-Lowry theory. Any reaction involving the formation of a coordinate covalent bond can be considered a Lewis acid-base reaction. This includes many reactions that don't involve proton transfer, such as the formation of complex ions and certain organic reactions.
Example: The reaction between BF₃ and NH₃:
BF₃ (Lewis acid) + NH₃ (Lewis base) → F₃B-NH₃
In this reaction, NH₃ donates its lone pair of electrons to the vacant orbital of BF₃, forming a coordinate covalent bond. This is a Lewis acid-base reaction, but it doesn't involve the transfer of a proton.
Key Differences Summarized:
| Feature | Brønsted-Lowry | Lewis |
|---|---|---|
| Definition | Proton transfer | Electron pair donation/acceptance |
| Acid | Proton donor | Electron pair acceptor |
| Base | Proton acceptor | Electron pair donor |
| Scope | Limited to proton transfer reactions | Includes a wider range of reactions |
| Examples of Acids | HCl, CH₃COOH, NH₄⁺ | BF₃, AlCl₃, transition metal ions |
| Examples of Bases | H₂O, NH₃, OH⁻ | NH₃, H₂O, Cl⁻ |
Applications and Significance:
Both Brønsted-Lowry and Lewis theories are essential tools in understanding chemical reactions. The choice of which theory to use depends on the specific reaction being considered.
-
Brønsted-Lowry theory: This theory is particularly useful for understanding acid-base reactions in aqueous solutions, such as titrations and buffer systems. It provides a clear framework for understanding acid strength and pH.
-
Lewis theory: This theory is more broadly applicable, encompassing a wider range of reactions, including those involving coordination compounds, organometallic chemistry, and many organic reactions. Understanding Lewis acid-base interactions is crucial in catalysis, materials science, and biochemistry.
Conclusion:
The Brønsted-Lowry and Lewis definitions of acids and bases offer complementary perspectives on acid-base chemistry. While the Brønsted-Lowry theory focuses on proton transfer, the Lewis theory provides a more general framework based on electron pair interactions. Both theories are essential for a comprehensive understanding of chemical reactivity, and their application depends on the specific context of the reaction being considered. Mastering both theories is vital for anyone pursuing a deeper understanding of chemistry. The ability to differentiate between and apply both models appropriately showcases a strong foundation in fundamental chemical principles. This understanding extends beyond classroom theory, proving crucial for advancements in various scientific fields that rely heavily on chemical reactions and interactions. From designing new catalysts to understanding biological processes, the concepts discussed here lay the groundwork for significant scientific breakthroughs.
Latest Posts
Latest Posts
-
Difference Between Reference And Thematic Maps
Mar 20, 2025
-
Which Change Of Phase Is Exothermic
Mar 20, 2025
-
What Elutes First In Gas Chromatography
Mar 20, 2025
-
Cross Section Of A Leaf Microscope
Mar 20, 2025
-
Are Gymnosperms Gametophyte Or Sporophyte Dominant
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Bronsted Lowry And Lewis . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
