Difference Between Intermolecular And Intramolecular Forces
Muz Play
Mar 25, 2025 · 6 min read
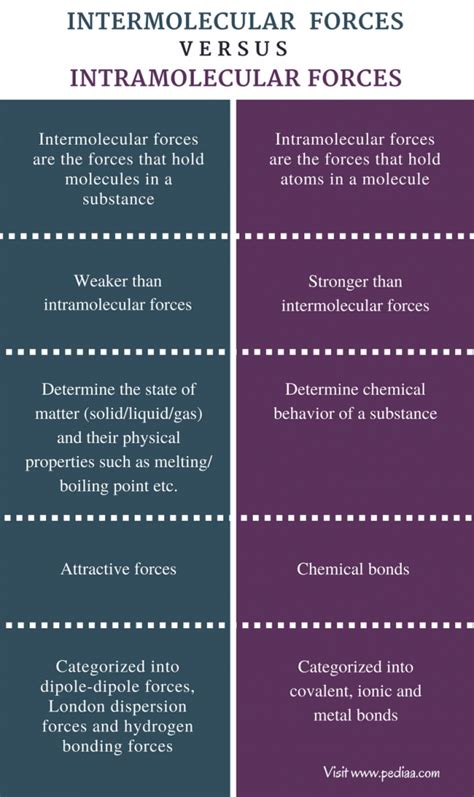
Table of Contents
Delving Deep: The Crucial Differences Between Intermolecular and Intramolecular Forces
Understanding the forces that govern the behavior of molecules is fundamental to chemistry. These forces, broadly categorized as intermolecular and intramolecular forces, dictate properties like melting point, boiling point, solubility, and reactivity. While often confused, the distinction between these two types of forces is crucial for comprehending the macroscopic world around us, from the fluidity of water to the strength of a diamond. This article will comprehensively explore the differences between intermolecular and intramolecular forces, providing detailed examples and explanations.
Intermolecular Forces: Interactions Between Molecules
Intermolecular forces (IMFs) are the attractive or repulsive forces between molecules. These forces are significantly weaker than the intramolecular forces (discussed below) that hold atoms together within a molecule. They are responsible for many of the physical properties of substances, particularly those in condensed phases (liquids and solids). The strength of IMFs determines the state of matter (solid, liquid, or gas) at a given temperature and pressure.
Types of Intermolecular Forces: A Hierarchical Overview
Intermolecular forces exist on a spectrum of strengths, with several distinct types:
1. London Dispersion Forces (LDFs): The Universal Force:
-
Mechanism: LDFs, also known as van der Waals forces, are the weakest type of IMF. They arise from temporary, instantaneous fluctuations in electron distribution around atoms or molecules. These fluctuations create temporary dipoles, which induce dipoles in neighboring molecules, leading to weak attractive forces. Even nonpolar molecules, which have no permanent dipole moment, exhibit LDFs.
-
Strength: LDF strength increases with the size and shape of the molecule. Larger molecules with more electrons have greater electron cloud polarizability, meaning their electron distribution is more easily distorted, leading to stronger LDFs. A longer, more extended shape also increases the contact surface area between molecules, enhancing LDFs.
-
Examples: LDFs are present in all molecules, but they are the only IMF present in nonpolar molecules like noble gases (He, Ne, Ar), hydrocarbons (methane, ethane), and diatomic nonpolar molecules (O₂, N₂).
2. Dipole-Dipole Forces: Attraction Between Polar Molecules:
-
Mechanism: Polar molecules possess a permanent dipole moment due to an uneven distribution of electrons caused by differences in electronegativity between atoms. The partially positive end of one polar molecule is attracted to the partially negative end of another, resulting in a dipole-dipole force.
-
Strength: Dipole-dipole forces are stronger than LDFs but weaker than hydrogen bonds. The strength depends on the magnitude of the dipole moment. Larger dipole moments lead to stronger interactions.
-
Examples: Molecules like HCl, HBr, and acetone exhibit dipole-dipole interactions in addition to LDFs.
3. Hydrogen Bonding: A Special Type of Dipole-Dipole Interaction:
-
Mechanism: Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (fluorine, oxygen, or nitrogen) interacts with a lone pair of electrons on another electronegative atom in a nearby molecule.
-
Strength: Hydrogen bonds are significantly stronger than typical dipole-dipole forces due to the high electronegativity of F, O, and N and the small size of the hydrogen atom, allowing for close proximity and strong electrostatic interactions.
-
Examples: Water (H₂O), ammonia (NH₃), and alcohols (e.g., methanol, ethanol) are classic examples where hydrogen bonding plays a crucial role in their properties. The high boiling point of water is a direct consequence of its strong hydrogen bonding network.
Intramolecular Forces: Bonds Within a Molecule
Intramolecular forces are the strong forces within a molecule that hold atoms together. These are the chemical bonds that determine the molecule's structure and reactivity. They are significantly stronger than intermolecular forces and are responsible for a molecule's chemical identity.
Types of Intramolecular Forces: The Building Blocks of Molecules
The principal types of intramolecular forces are:
1. Covalent Bonds:
-
Mechanism: Covalent bonds form when atoms share electrons to achieve a stable electron configuration (often an octet). This sharing creates a strong attractive force between the atoms.
-
Strength: Covalent bonds vary in strength depending on the atoms involved and the bond order (single, double, or triple bond). Generally, they are much stronger than intermolecular forces.
-
Examples: The bonds in water (O-H), methane (C-H), and ethene (C=C) are all covalent bonds.
2. Ionic Bonds:
-
Mechanism: Ionic bonds form when one atom transfers an electron (or electrons) to another atom. This creates ions (cations and anions) with opposite charges that are strongly attracted to each other through electrostatic forces.
-
Strength: Ionic bonds are generally stronger than covalent bonds, although their strength can vary depending on the charges and sizes of the ions involved.
-
Examples: Sodium chloride (NaCl), magnesium oxide (MgO), and potassium iodide (KI) are all examples of compounds with ionic bonds.
3. Metallic Bonds:
-
Mechanism: Metallic bonds are found in metals. In this case, valence electrons are delocalized and shared amongst a "sea" of electrons, creating a strong attractive force that holds the metal atoms together.
-
Strength: The strength of metallic bonds varies depending on the metal. The strength is often related to the number of valence electrons and the atomic radius of the metal atoms.
-
Examples: The bonds in copper (Cu), iron (Fe), and aluminum (Al) are all metallic bonds.
Key Differences Summarized: A Comparative Table
| Feature | Intermolecular Forces | Intramolecular Forces |
|---|---|---|
| Nature | Forces between molecules | Forces within molecules |
| Strength | Weak | Strong |
| Type of Interaction | Electrostatic, van der Waals | Covalent, ionic, metallic |
| Effect on Properties | Boiling point, melting point, solubility, viscosity | Chemical reactivity, bond angles, bond lengths |
| Examples | LDFs, dipole-dipole, hydrogen bonds | Bonds in water, NaCl, metals |
| Broken during | Phase transitions (melting, boiling) | Chemical reactions |
Real-World Applications and Implications
The difference between intermolecular and intramolecular forces is crucial in understanding a wide range of phenomena:
-
Solubility: The solubility of a substance is heavily influenced by the interplay of intermolecular forces between the solute and the solvent. "Like dissolves like" – polar solvents dissolve polar solutes (due to dipole-dipole interactions and hydrogen bonding), while nonpolar solvents dissolve nonpolar solutes (due to LDFs).
-
Boiling Point: The boiling point of a liquid is the temperature at which the intermolecular forces are overcome, allowing molecules to escape into the gaseous phase. Stronger IMFs result in higher boiling points.
-
Surface Tension: Surface tension is a consequence of the imbalance of intermolecular forces at the surface of a liquid. Molecules at the surface experience a net inward force, leading to a minimized surface area.
-
Viscosity: Viscosity, or the resistance of a liquid to flow, is influenced by the strength of intermolecular forces. Stronger IMFs lead to higher viscosity.
-
Chemical Reactions: Intramolecular forces determine the chemical behavior of molecules. Breaking and forming chemical bonds (intramolecular forces) are central to all chemical reactions.
Conclusion: Understanding the Force Balance
The distinction between intermolecular and intramolecular forces is paramount in understanding the physical and chemical properties of matter. While intramolecular forces define the structure and reactivity of molecules, intermolecular forces govern the interactions between these molecules, dictating the macroscopic properties we observe. This fundamental understanding is vital not only for advanced studies in chemistry but also for advancements in various fields, including materials science, pharmaceuticals, and nanotechnology. By appreciating the strength and nature of both intermolecular and intramolecular forces, we gain a deeper appreciation for the intricate complexity of the world around us.
Latest Posts
Latest Posts
-
What Is Periodic Motion In Physics
Mar 27, 2025
-
How To Factor Trinomials With Leading Coefficient
Mar 27, 2025
-
How Much Atp Is Produced In Oxidative Phosphorylation
Mar 27, 2025
-
Definition Of Uniform Motion In Physics
Mar 27, 2025
-
Why Is A Cells Size Limited
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Intermolecular And Intramolecular Forces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
