Difference Between Open And Closed System
Muz Play
Mar 25, 2025 · 7 min read
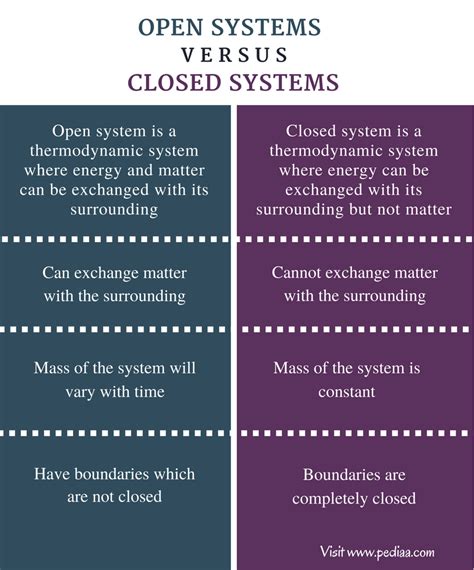
Table of Contents
Delving Deep into the Differences: Open vs. Closed Systems
The concepts of open and closed systems are fundamental across numerous scientific disciplines, from physics and chemistry to ecology and computer science. Understanding the distinctions between these system types is crucial for analyzing complex phenomena and building effective models. While seemingly simple at first glance, the nuances of each system type can be surprisingly intricate. This comprehensive guide will explore the core differences between open and closed systems, providing detailed examples and highlighting their practical implications.
Defining Open and Closed Systems: A Foundational Overview
Before diving into the specifics, let's establish clear definitions. A system, in its broadest sense, is a collection of interconnected components working together as a whole. These components interact and influence each other, exhibiting emergent properties not readily apparent by examining individual parts.
An open system readily exchanges both energy and matter with its surroundings. Think of it as a system with permeable boundaries; resources flow freely in and out. Conversely, a closed system exchanges only energy with its environment. Matter remains within the system's boundaries; it doesn't enter or leave. It's important to note that the concept of a truly isolated system – one that exchanges neither energy nor matter – is largely theoretical; such systems are exceedingly rare in the real world.
Key Distinguishing Factors: A Comparative Analysis
| Feature | Open System | Closed System |
|---|---|---|
| Matter Exchange | Exchanges matter with its surroundings | No matter exchange with surroundings |
| Energy Exchange | Exchanges energy with its surroundings | Exchanges energy with its surroundings |
| Entropy | Can maintain low entropy through exchange | Entropy tends to increase over time |
| Examples | Human body, ecosystem, a boiling pot of water | A sealed container of gas, a thermos flask (approximately) |
| Equilibrium | Dynamic equilibrium (constant flux) | Tends towards thermodynamic equilibrium |
| Complexity | Typically more complex and dynamic | Can be simpler, but still complex |
Open Systems: A World of Interconnectedness
Open systems are characterized by constant interaction with their environment. This interaction drives their dynamic behavior and influences their evolution over time. The continuous flow of matter and energy creates a state of dynamic equilibrium, where inputs and outputs balance, maintaining a relatively stable state despite the constant flux. Let's explore some detailed examples:
1. The Human Body: A Complex Open System
The human body is a prime example of an open system. We constantly exchange matter (food, water, oxygen) and energy (heat, work) with our surroundings. We ingest nutrients, absorb oxygen, and excrete waste products. We also generate heat and perform work. The body's internal processes work to maintain homeostasis, a relatively stable internal environment despite external fluctuations. This demonstrates the dynamic equilibrium characteristic of open systems.
2. Ecosystems: A Web of Interacting Components
Ecosystems, comprising living organisms and their physical environment, are quintessential open systems. Energy flows through the ecosystem through producers (plants), consumers (animals), and decomposers. Matter cycles through the system, with nutrients being taken up by organisms and eventually returned to the environment through decomposition. The continuous exchange of both matter and energy sustains the ecosystem's biodiversity and functionality. Climate change, pollution, and resource depletion represent disruptions to this flow, highlighting the vulnerability of open systems to external pressures.
3. Boiling Water: A Simpler Open System Illustration
Even a seemingly simple process like boiling water in an open pot exemplifies an open system. Heat energy is added (input), causing water molecules to gain kinetic energy and transition from liquid to gas. Water vapor (matter) escapes into the atmosphere (output). The continuous energy input and matter output maintain the boiling process until the water is depleted.
Closed Systems: Conservation and Equilibrium
In contrast to open systems, closed systems are characterized by the conservation of matter within their boundaries. While energy can be exchanged with the surroundings, the amount of matter remains constant. This constraint influences the system's behavior, leading to a tendency toward thermodynamic equilibrium, a state of maximum entropy (disorder) within the constraints of the system.
1. A Sealed Container of Gas: A Classic Closed System
Imagine a sealed container filled with a gas. The gas molecules interact, colliding and transferring energy. If the container is heated (energy input), the gas molecules will move faster, increasing the pressure inside. However, no matter enters or leaves the container. Eventually, if the heating is stopped, the system will reach a state of thermodynamic equilibrium, where the temperature and pressure within the container are uniform.
2. A Thermos Flask (Approximation): Limited Exchange
A thermos flask, designed to minimize heat transfer, approximates a closed system. Although it's not perfectly sealed (some minor heat exchange occurs), the flask significantly restricts the transfer of both matter and energy. The aim is to maintain the temperature of the contents (be it hot or cold) for an extended period. The longer the temperature stays stable, the closer it approximates the ideal of a closed system. However, over time, even a thermos flask will fail to maintain a constant temperature and the system will reach equilibrium with the external environment.
3. The Earth's Atmosphere (Simplified): A Complex Example
While the Earth's atmosphere is a complex system with significant open aspects (e.g., the exchange of gases with space through volcanic eruptions and meteor showers), considering it as a partially closed system with respect to certain gases can be a useful approximation for certain models. For instance, analyzing the carbon cycle, focusing only on the exchange of carbon dioxide between the atmosphere and other Earth systems (like oceans and biosphere) and disregarding outside sources (e.g. from outer space) is a simplified approach employing the closed system concept.
Implications and Applications: Real-World Significance
Understanding the distinction between open and closed systems is crucial in various fields. For example, in ecology, recognizing the open nature of ecosystems allows us to analyze the impacts of pollution, climate change, and habitat destruction on biodiversity and resource availability. In engineering, it dictates the design and operation of systems ranging from chemical reactors to power plants. The principles of open and closed systems form the basis of many scientific models and simulations, allowing us to predict and interpret complex phenomena.
Furthermore, the open vs. closed system dichotomy finds applications in systems thinking, a holistic approach to problem-solving. Recognizing whether a system is open or closed is a crucial first step in determining appropriate strategies for managing and controlling the system. Open systems require considering the external environment and its influence on the system’s behavior. Closed systems, while less susceptible to external influences, may still exhibit complex internal dynamics.
For instance, designing a sustainable city requires understanding it as a complex open system with continuous interactions with its surrounding environment. Similarly, developing effective climate change policies demands a comprehensive understanding of the Earth's climate as a multifaceted open system where changes in one component impact others in a global network of cause-and-effect relationships.
Conclusion: A Continuous Spectrum
While the open and closed system dichotomy provides a valuable framework for understanding system behavior, it's crucial to acknowledge that the line between these categories isn't always sharply defined. Many systems exhibit characteristics of both open and closed systems depending on the scale and context of the analysis. For example, a cell might be considered an open system in its exchange with its surrounding fluids, but a relatively closed system with respect to certain internal processes. A thorough analysis often requires considering the system's boundaries and the nature of its interactions with its surroundings, recognizing the potential for a spectrum of openness rather than a strict binary classification. By understanding these nuances, we gain a more powerful and versatile tool for modeling and interpreting the complex world around us.
Latest Posts
Latest Posts
-
Chemical Equilibrium Le Chateliers Principle Lab Answers
Mar 26, 2025
-
Peroxisomes Got Their Name Because Hydrogen Peroxide Is
Mar 26, 2025
-
State Of Matter With Definite Shape And Volume
Mar 26, 2025
-
Question Lexan Draw The Monomer Used To Make This Polymer
Mar 26, 2025
-
How Do You Calculate Index Numbers
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Open And Closed System . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
