Difference Between Thin Layer Chromatography And Column Chromatography
Muz Play
Mar 17, 2025 · 6 min read
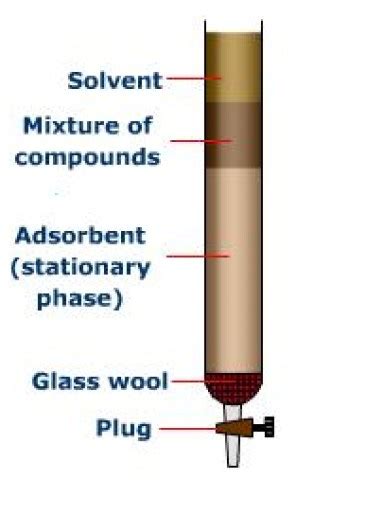
Table of Contents
Thin Layer Chromatography vs. Column Chromatography: A Comprehensive Comparison
Chromatography, a cornerstone technique in analytical chemistry, boasts a diverse range of applications, from separating complex mixtures to purifying individual compounds. Among its many variations, thin-layer chromatography (TLC) and column chromatography stand out as fundamental methods. While both leverage the principle of differential adsorption to separate components, they differ significantly in their setup, scale, application, and analytical capabilities. This comprehensive guide delves into the nuances of TLC and column chromatography, highlighting their similarities and differences to help you choose the optimal technique for your specific needs.
Understanding the Principles: Differential Adsorption
At the heart of both TLC and column chromatography lies the principle of differential adsorption. This principle exploits the varying affinities of different components within a mixture for both a stationary phase (a solid material) and a mobile phase (a liquid or gas). The stationary phase is typically a porous material like silica gel or alumina, while the mobile phase is a solvent or a mixture of solvents.
Components with a stronger affinity for the stationary phase will move more slowly through the chromatographic system, while those with a stronger affinity for the mobile phase will move more quickly. This differential migration separates the mixture's components into distinct bands or zones.
Thin Layer Chromatography (TLC): A Quick and Simple Technique
TLC is a widely used, planar chromatographic technique characterized by its simplicity, speed, and low cost. It involves spotting a small amount of the sample onto a thin layer of adsorbent material, usually silica gel or alumina, coated on a glass or plastic plate. The plate is then placed in a developing chamber containing a suitable mobile phase. As the mobile phase ascends the plate by capillary action, the components of the sample separate based on their differential affinities for the stationary and mobile phases.
Advantages of TLC:
- Speed and Simplicity: TLC is remarkably fast and easy to perform, making it ideal for quick analyses and preliminary investigations.
- Low Cost: The materials and equipment required for TLC are relatively inexpensive.
- Versatility: TLC can be used to separate a wide range of compounds, including organic molecules, inorganic ions, and even biological macromolecules.
- Qualitative Analysis: TLC is primarily a qualitative technique, providing information on the number of components in a mixture and their relative polarities.
- Visualization: Separated components can be visualized using various techniques, such as UV light, iodine vapor, or specific chemical stains. This allows for easy identification and comparison.
Disadvantages of TLC:
- Limited Resolution: Compared to column chromatography, TLC offers lower resolution, making it less suitable for separating complex mixtures with closely related components.
- Small Sample Size: TLC can only handle small quantities of sample, limiting its applicability for preparative purposes.
- Semi-quantitative Analysis: While not inherently quantitative, densitometry can be used to obtain semi-quantitative data from TLC plates. However, this is less precise than methods used with column chromatography.
- Susceptibility to Errors: Factors like the quality of the TLC plate, the solvent system, and the development conditions can significantly influence the separation and reproducibility of results.
Column Chromatography: A Powerful Purification Technique
Column chromatography, on the other hand, is a three-dimensional technique used for both analytical and preparative purposes. It involves packing a glass or plastic column with a stationary phase, typically silica gel or alumina, and then carefully adding the sample mixture to the top of the column. A mobile phase is then passed through the column, typically by gravity or under pressure. As the mobile phase flows through the column, the components of the sample separate based on their differential affinities for the stationary and mobile phases, eluting from the column at different times.
Advantages of Column Chromatography:
- High Resolution: Column chromatography offers significantly higher resolution than TLC, allowing for the separation of complex mixtures with closely related components.
- Larger Sample Capacity: Column chromatography can handle much larger quantities of sample than TLC, making it ideal for preparative purposes—isolating pure compounds for further analysis or use.
- Quantitative Analysis: The amount of each component eluting from the column can be quantified using various techniques such as UV-Vis spectroscopy, HPLC detectors, or other methods. This provides precise information on the composition of the mixture.
- Fraction Collection: Individual components can be collected as they elute from the column, yielding purified samples of each component.
Disadvantages of Column Chromatography:
- Time-Consuming: Column chromatography is generally a more time-consuming technique than TLC, requiring careful packing of the column and gradual elution of the components.
- Higher Cost: The materials and equipment needed for column chromatography are typically more expensive than those for TLC.
- Technical Expertise: Proper column packing and elution require a higher level of technical expertise compared to TLC.
- Solvent Waste: Column chromatography often involves the use of significant amounts of solvent, which can be a disadvantage from an environmental perspective.
Head-to-Head Comparison: TLC vs. Column Chromatography
| Feature | Thin Layer Chromatography (TLC) | Column Chromatography |
|---|---|---|
| Scale | Microscale | Macroscale to Preparative Scale |
| Resolution | Low | High |
| Speed | Fast | Slow |
| Cost | Low | Moderate to High |
| Sample Size | Small | Large |
| Application | Qualitative analysis, preliminary separation | Qualitative & quantitative analysis, purification |
| Complexity | Simple | More complex |
| Automation | Limited | High (e.g. HPLC) |
| Fraction Collection | Not applicable | Applicable |
Choosing the Right Technique: A Practical Guide
The choice between TLC and column chromatography depends primarily on the specific application and the desired outcome.
-
Use TLC for:
- Quick screening: Determining the number and approximate polarity of components in a mixture.
- Monitoring reaction progress: Tracking the disappearance of reactants or the appearance of products.
- Optimizing chromatographic conditions: Identifying a suitable solvent system for column chromatography.
- Simple separations: Separating mixtures with distinct components.
-
Use column chromatography for:
- Purification of compounds: Isolating pure compounds from complex mixtures.
- Quantitative analysis: Determining the precise amount of each component in a mixture.
- Preparative separations: Obtaining larger quantities of pure compounds.
- Separations requiring high resolution: Separating closely related compounds.
Advanced Techniques and Hybrid Approaches
The field of chromatography continues to evolve, with new techniques and hybrid approaches constantly emerging. High-performance liquid chromatography (HPLC) is a sophisticated form of column chromatography that utilizes high pressure to enhance separation efficiency and speed. Flash column chromatography is a faster variation of traditional column chromatography employing positive pressure to accelerate the elution process. These advanced techniques often incorporate sophisticated detectors and data acquisition systems for enhanced quantitative analysis and automation.
Conclusion
Both thin-layer chromatography and column chromatography are invaluable tools in the chemist's arsenal. TLC serves as a rapid, inexpensive method for preliminary analysis and qualitative assessments, while column chromatography excels in its ability to separate complex mixtures and purify individual components on a preparative scale. The choice between these techniques depends on the specific requirements of the application, the complexity of the mixture being analyzed, and the resources available. By understanding the strengths and limitations of each technique, researchers can effectively leverage the power of chromatography to advance their scientific endeavors.
Latest Posts
Latest Posts
-
Do Seedless Vascular Plants Need Water For Fertilization
Mar 17, 2025
-
The Fmet Trna Differs From The Met Trna In That
Mar 17, 2025
-
Are Cilia And Flagella Microtubules Or Microfilaments
Mar 17, 2025
-
Why Activation Energy Is Not Affected By Temperature
Mar 17, 2025
-
Thiols Have Structures Similar To Alcohols Except That They Contain
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Thin Layer Chromatography And Column Chromatography . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
