Thiols Have Structures Similar To Alcohols Except That They Contain
Muz Play
Mar 17, 2025 · 6 min read
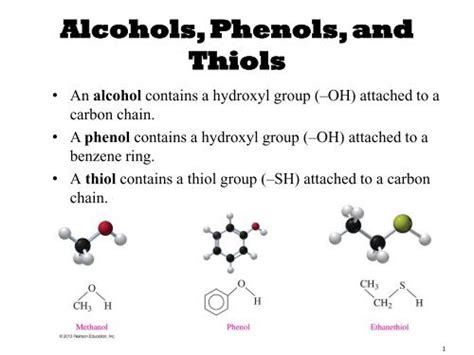
Table of Contents
Thiols: The Sulfur Siblings of Alcohols – Structure, Properties, and Applications
Thiols, also known as mercaptans, are organic sulfur compounds that bear a striking resemblance to their oxygenated counterparts, alcohols. The key difference lies in the replacement of the hydroxyl (-OH) group in alcohols with a sulfhydryl (-SH) group. This seemingly simple substitution, however, leads to a dramatic shift in the chemical and physical properties of these compounds, opening up a vast array of applications across various fields. This comprehensive article delves into the structural similarities and differences between thiols and alcohols, explores their unique properties, and highlights their significant roles in various industries.
Structural Similarities and Differences: The Heart of the Matter
At the core of understanding thiols lies the comparison with their alcohol analogs. Both alcohols and thiols are characterized by a functional group attached to a carbon atom. In alcohols, this functional group is the hydroxyl (-OH) group, while in thiols, it's the sulfhydryl (-SH) group. This seemingly minor alteration significantly impacts the molecule's behavior.
The Hydroxyl (-OH) vs. the Sulfhydryl (-SH) Group: A Tale of Two Groups
The difference between oxygen and sulfur is crucial. Oxygen is significantly more electronegative than sulfur. This difference in electronegativity influences the polarity of the bonds and consequently the overall properties of the molecules. The O-H bond in alcohols is highly polar, leading to strong hydrogen bonding between alcohol molecules. This strong hydrogen bonding results in higher boiling points and increased solubility in water compared to their corresponding thiols.
Conversely, the S-H bond in thiols is less polar due to the lower electronegativity of sulfur. This weaker polarity leads to weaker intermolecular forces, resulting in lower boiling points and generally lower solubility in water than their alcohol counterparts. This difference in polarity also impacts their reactivity.
Nomenclature: Naming the Players
The nomenclature of thiols is closely related to that of alcohols. The name of a thiol is derived from the corresponding alkane by replacing the "-e" ending with "-thiol." For example, CH₃SH is called methanethiol, while CH₃CH₂SH is ethanethiol. Simple thiols often have common names, some of which are still widely used, for instance, the pungent smell associated with many thiols is responsible for the common name "mercaptan," stemming from the Latin "mercurium captans," meaning "mercury capturing," referencing their ability to form stable complexes with mercury.
Physical and Chemical Properties: A World Apart
While structurally similar, the physical and chemical properties of thiols and alcohols differ significantly. These differences stem primarily from the distinct electronegativities of oxygen and sulfur, impacting their bonding, polarity, and reactivity.
Boiling Points and Solubility: The Impact of Intermolecular Forces
As mentioned earlier, the weaker intermolecular forces in thiols result in significantly lower boiling points compared to their alcohol counterparts. This is because the S-H bond is less polar, leading to weaker hydrogen bonding and van der Waals forces. Similarly, thiols exhibit lower solubility in water due to the reduced polarity and the inability to form strong hydrogen bonds with water molecules. Higher molecular weight thiols are largely insoluble in water.
Acidity: The Proton's Journey
One of the most notable differences lies in their acidity. Thiols are significantly more acidic than alcohols. This increased acidity is attributed to the larger size of the sulfur atom compared to oxygen. The larger size results in a weaker S-H bond, making it easier to release a proton (H⁺) and form a thiolate anion (RS⁻). This enhanced acidity allows thiols to react with bases more readily than alcohols. This increased acidity plays a significant role in many of their applications.
Oxidation: A Reactive Tale
Thiols are readily oxidized, often forming disulfides (R-S-S-R). This oxidation process involves the removal of two hydrogen atoms from two thiol molecules, resulting in the formation of a disulfide bond. Disulfide bonds are crucial in determining the tertiary structure of proteins, and their formation and breakage are vital biological processes. Conversely, alcohols undergo oxidation to form aldehydes or ketones, a fundamentally different reaction pathway.
Reactivity and Reactions: Exploring the Chemical Landscape
Thiols demonstrate a rich chemistry, exhibiting various reactions characteristic of their sulfhydryl group. These reactions are distinct from those of alcohols and underlie many of their applications.
Nucleophilic Attacks: The Thiol's Offensive
The sulfur atom in the -SH group is a relatively good nucleophile, meaning it readily donates a pair of electrons to form a new bond. This nucleophilicity allows thiols to participate in a wide range of nucleophilic substitution and addition reactions. This is a significant difference from alcohols, where the oxygen atom is a less effective nucleophile.
Reaction with Heavy Metals: Mercaptan's Affinity
As mentioned earlier, thiols readily react with heavy metals, forming stable complexes. This property was the basis for the common name "mercaptan," highlighting their ability to "capture" mercury. This interaction is exploited in various applications, including detoxification of heavy metal poisoning and in the extraction of heavy metals from industrial waste.
Formation of Disulfides: The Bond that Binds
The oxidation of thiols to form disulfides is a crucial reaction, especially in biological systems. Disulfide bonds are essential for maintaining the three-dimensional structure of proteins. The formation and cleavage of disulfide bonds are involved in a range of biological processes, including protein folding and enzymatic activity.
Esterification: A Sulfur Twist
Thiols can undergo esterification reactions, similar to alcohols, but forming thioesters instead of esters. Thioesters are important intermediates in many biological processes, particularly in metabolism and energy transfer.
Applications: A Multifaceted Role
Thiols and their derivatives find wide-ranging applications across various fields, leveraging their unique chemical properties.
Biological Significance: Life's Essential Sulfur
Thiol groups play essential roles in biological systems. Cysteine, an amino acid containing a thiol group, is a critical component of proteins. The disulfide bonds formed between cysteine residues contribute significantly to protein structure and function. Coenzyme A, a vital molecule involved in metabolism, also incorporates a thiol group. Glutathione, a powerful antioxidant, is a tripeptide containing a thiol group, crucial for cellular detoxification.
Industrial Applications: From Smell to Synthesis
Thiols are used as odorants, especially in the natural gas industry. The strong and unpleasant smell of methanethiol (methyl mercaptan) is added to odorless natural gas to facilitate the detection of leaks, preventing potentially hazardous situations. Various thiols and their derivatives are also used as intermediates in the synthesis of other organic compounds and in the production of pharmaceuticals.
Medical Applications: Targeting the Thiol
Thiols are involved in various medical applications. Some thiols have antioxidant properties and are being explored for their potential in preventing or treating certain diseases. Additionally, the high reactivity of thiols makes them useful in the design of drugs and diagnostic tools, often targeting specific thiol groups within proteins or other biomolecules.
Environmental Applications: Cleaning Up
The ability of thiols to react with heavy metals has led to their use in environmental remediation, helping in the removal of heavy metal pollutants from contaminated water and soil.
Conclusion: A Broad Spectrum of Possibilities
Thiols, while structurally similar to alcohols, exhibit a remarkably distinct set of properties and reactivities. Their unique characteristics, driven by the replacement of oxygen with sulfur, open up a wide array of applications in biology, industry, medicine, and environmental science. From the pungent smell added to natural gas to the critical roles they play in protein structure and function, thiols' multifaceted nature continues to fascinate and inspire research and innovation across various fields. Further research into their reactivity and applications promises to reveal even more of their potential in shaping our future.
Latest Posts
Latest Posts
-
Identify The Equation For The Graph
Mar 17, 2025
-
Ions With Positive Charge Are Called
Mar 17, 2025
-
What Is A Derived Unit In Chemistry
Mar 17, 2025
-
What Does The Coefficient Represent In A Chemical Formula
Mar 17, 2025
-
How To Calculate Saturated Vapour Pressure
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Thiols Have Structures Similar To Alcohols Except That They Contain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
