Do Acids Or Base React With Metals
Muz Play
Mar 20, 2025 · 6 min read
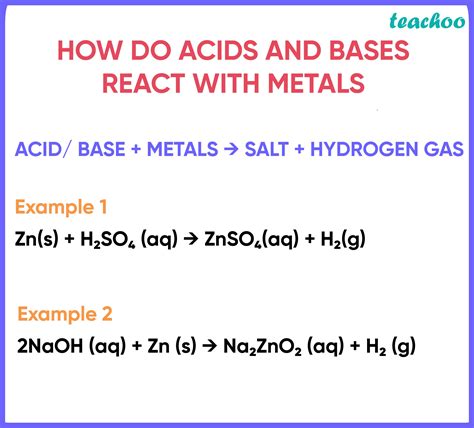
Table of Contents
Do Acids or Bases React with Metals? A Comprehensive Exploration
The reactivity of metals with acids and bases is a fundamental concept in chemistry, with significant implications in various fields, from industrial processes to biological systems. While the general rule of thumb states that acids react with metals, the reality is more nuanced and fascinating. This comprehensive exploration delves into the details of metal-acid and metal-base reactions, examining the factors that influence reactivity, specific examples, and the underlying chemical principles.
Understanding Metal Reactivity
Before diving into the reactions themselves, it's crucial to understand the inherent properties of metals that govern their reactivity. Metals possess a characteristic tendency to lose electrons, a property known as electropositivity. This ability to readily donate electrons determines how readily a metal will participate in a chemical reaction. The electrochemical series, a table ranking metals based on their standard reduction potentials, provides a valuable tool for predicting the relative reactivity of different metals. Metals higher on this series are more electropositive and therefore more reactive.
Factors Affecting Metal Reactivity
Several factors influence the reactivity of a metal, beyond its inherent electropositivity:
-
Concentration of the acid or base: A higher concentration of the reactant generally leads to a faster reaction rate. More reactant particles mean more frequent collisions and a higher probability of successful reactions.
-
Temperature: Increasing the temperature typically accelerates the reaction rate. Higher temperatures provide the reacting particles with greater kinetic energy, increasing the frequency and effectiveness of collisions.
-
Surface area of the metal: A larger surface area exposed to the reactant allows for more contact points, increasing the rate of reaction. Finely divided metal powders react much faster than solid chunks of the same metal.
-
Presence of impurities: Impurities on the metal surface can either catalyze or inhibit the reaction, affecting the overall rate.
-
Nature of the acid or base: Different acids and bases possess varying strengths and reactivities, directly impacting the speed and extent of the reaction with a metal. Strong acids like hydrochloric acid (HCl) and sulfuric acid (H₂SO₄) typically react more vigorously than weak acids like acetic acid (CH₃COOH). Similarly, strong bases react more readily than weak bases.
Metal-Acid Reactions: The Fundamentals
The most common and widely studied reactions involving metals are those with acids. These reactions generally produce a salt and hydrogen gas (H₂) as a byproduct. The general equation for a metal reacting with an acid can be represented as:
Metal + Acid → Salt + Hydrogen Gas
For example, the reaction of zinc with hydrochloric acid is:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
This reaction is characterized by the evolution of hydrogen gas, which can be observed as bubbling. The salt formed, in this case zinc chloride, remains dissolved in the solution.
Not All Metals React with Acids
It's crucial to emphasize that not all metals react with all acids. The reactivity depends on the position of the metal in the electrochemical series and the strength of the acid. Noble metals, such as gold (Au) and platinum (Pt), are extremely unreactive and do not react with most common acids. These metals have very high reduction potentials and are not easily oxidized.
The Role of Oxidation and Reduction
Metal-acid reactions are examples of redox reactions, involving both oxidation and reduction processes. The metal is oxidized, losing electrons to form a positive ion, while the hydrogen ions in the acid are reduced, gaining electrons to form hydrogen gas. The transfer of electrons is the driving force behind the reaction.
Metal-Base Reactions: A Less Common but Significant Interaction
While less prevalent than metal-acid reactions, certain metals, particularly those that are amphiprotic (capable of acting as both acids and bases), can react with strong bases. These reactions typically involve the formation of a complex ion and the evolution of hydrogen gas.
The reaction of aluminum with a strong base like sodium hydroxide (NaOH) serves as a classic example:
2Al(s) + 2NaOH(aq) + 6H₂O(l) → 2Na + 3H₂(g)
In this reaction, aluminum reacts with sodium hydroxide and water to produce sodium tetrahydroxoaluminate(III) and hydrogen gas. This reaction demonstrates the amphoteric nature of aluminum, as it reacts with both acids and bases.
Conditions Favoring Metal-Base Reactions
Metal-base reactions are often dependent on specific conditions. The presence of water is often essential, and the base needs to be sufficiently concentrated and strong. Furthermore, the metal's surface needs to be clean and free from oxides to ensure effective interaction with the base.
Comparing Metal-Acid and Metal-Base Reactivity
It's useful to compare and contrast metal-acid and metal-base reactions:
| Feature | Metal-Acid Reaction | Metal-Base Reaction |
|---|---|---|
| Frequency | More common | Less common |
| Metals Involved | Wide range, excluding noble metals | Typically amphoteric metals |
| Products | Salt + Hydrogen gas | Complex ion + Hydrogen gas |
| Reaction Conditions | Less stringent | Often requires water and strong base |
| Driving Force | Oxidation of metal, reduction of H⁺ ions | Oxidation of metal, formation of complex ion |
Practical Applications and Industrial Significance
The reactions of metals with acids and bases have widespread practical applications across various industries:
-
Metal Refining: Acid leaching is a crucial step in the extraction and purification of many metals from their ores. Acids are used to dissolve metal compounds, separating them from unwanted impurities.
-
Production of Hydrogen Gas: Metal-acid reactions are a common laboratory method for producing hydrogen gas, albeit not always the most efficient or environmentally friendly.
-
Cleaning and Etching of Metals: Acids are utilized in various cleaning and etching processes to remove surface oxides and impurities from metal surfaces.
-
Wastewater Treatment: Metal-base reactions can play a role in the treatment of wastewater containing heavy metal ions, converting them into less harmful forms.
-
Synthesis of Metal Compounds: Reactions between metals, acids, and bases are essential in the synthesis of various metal compounds used in diverse applications.
Safety Precautions
Working with acids and bases requires stringent safety precautions. Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and lab coats. Reactions can be exothermic, generating heat and potentially causing burns. Hydrogen gas produced in these reactions is highly flammable and should be handled with care in a well-ventilated area. Proper disposal of chemical waste is crucial to prevent environmental contamination.
Conclusion
The reactivity of metals with acids and bases is a complex topic governed by several factors, including the inherent properties of the metal, the strength of the acid or base, and the reaction conditions. While acids generally react more readily with metals, certain amphoteric metals can also react with strong bases. These reactions have wide-ranging industrial and scientific applications, but safety precautions should always be observed when working with these chemicals. Understanding the underlying principles of these reactions is crucial for anyone working in chemistry, materials science, or related fields. Further research into specific metal-acid and metal-base reactions is encouraged to deepen one's understanding of this fundamental area of chemistry.
Latest Posts
Latest Posts
-
How To Calculate The Average Mass
Mar 21, 2025
-
Is Viscosity A Physical Or Chemical Property
Mar 21, 2025
-
How Do You Calculate The Rate Of Diffusion
Mar 21, 2025
-
What Is A Unique Property Of Water
Mar 21, 2025
-
In A Phospholipid Molecule The Head Is
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Do Acids Or Base React With Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
