How To Calculate The Average Mass
Muz Play
Mar 21, 2025 · 5 min read
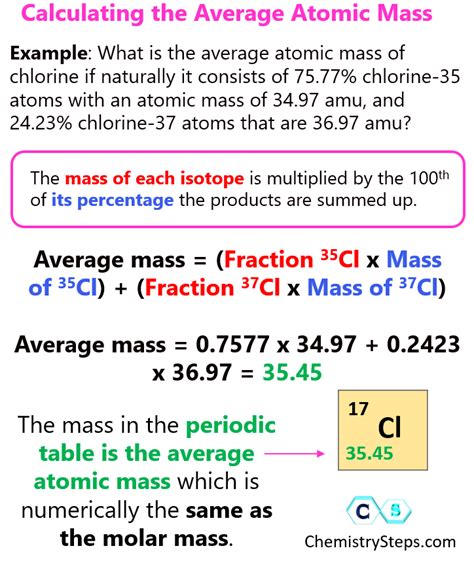
Table of Contents
How to Calculate Average Mass: A Comprehensive Guide
Calculating average mass is a fundamental concept with applications across numerous fields, from chemistry and physics to statistics and data analysis. Understanding how to calculate and interpret average mass is crucial for accurate scientific measurements, data analysis, and problem-solving. This comprehensive guide will delve into various methods for calculating average mass, exploring different scenarios and providing practical examples.
Understanding Mass and Average Mass
Before diving into the calculations, let's clarify the concepts of mass and average mass.
Mass: Mass is a fundamental property of matter that represents the amount of matter in an object. It's often measured in kilograms (kg) or grams (g), although other units may be used depending on the context. Unlike weight, mass remains constant regardless of location.
Average Mass: When dealing with multiple objects or samples with varying masses, we often need to determine the average mass. The average mass represents the central tendency of the mass distribution, providing a single representative value for the entire set. This value is particularly useful for simplifying calculations or making comparisons across different groups.
Methods for Calculating Average Mass
There are several ways to calculate average mass, depending on the type of data available and the specific requirements of the calculation.
1. Simple Arithmetic Mean: The Most Common Method
The simplest and most widely used method is the arithmetic mean. This involves summing the individual masses and dividing by the total number of objects or samples.
Formula:
Average Mass = (Sum of individual masses) / (Total number of objects)
Example:
Let's say you have three objects with masses of 10g, 15g, and 20g. To calculate the average mass:
Average Mass = (10g + 15g + 20g) / 3 = 15g
This method is straightforward and effective when dealing with a relatively small number of objects with similar masses.
2. Weighted Average Mass: Accounting for Different Frequencies
When dealing with data where different masses occur with varying frequencies, the simple arithmetic mean may not be sufficient. In such cases, a weighted average mass is more appropriate. This method takes into account the frequency of each mass value.
Formula:
Weighted Average Mass = Σ (massᵢ * frequencyᵢ) / Σ frequencyᵢ
Where:
- massᵢ = the mass of the i-th object
- frequencyᵢ = the number of times the i-th mass occurs
Example:
Imagine you have a collection of marbles: 5 marbles weighing 2g each, 3 marbles weighing 3g each, and 2 marbles weighing 4g each. The weighted average mass would be calculated as follows:
Weighted Average Mass = ((2g * 5) + (3g * 3) + (4g * 2)) / (5 + 3 + 2) = 2.7g
This method is crucial when dealing with larger datasets or when the frequency of different masses significantly impacts the overall average.
3. Average Mass from Density and Volume: For Homogeneous Substances
If you know the density and volume of a homogeneous substance (a substance with uniform composition throughout), you can calculate its average mass.
Formula:
Average Mass = Density * Volume
Example:
If a block of aluminum has a density of 2.7 g/cm³ and a volume of 10 cm³, its mass is:
Average Mass = 2.7 g/cm³ * 10 cm³ = 27g
This method is particularly useful when dealing with liquids or solids where direct mass measurement might be challenging.
4. Average Mass from Molecular Weight and Number of Moles: In Chemistry
In chemistry, the average mass of a substance can be calculated using its molecular weight (molar mass) and the number of moles.
Formula:
Average Mass = Molecular Weight * Number of Moles
Example:
The molecular weight of water (H₂O) is approximately 18 g/mol. If you have 2 moles of water, the average mass would be:
Average Mass = 18 g/mol * 2 mol = 36g
This method is fundamental in stoichiometric calculations and chemical analysis.
Advanced Considerations and Challenges
While the methods outlined above are generally straightforward, certain scenarios can introduce complexities:
Dealing with Uncertainties and Errors
In real-world measurements, uncertainties and errors are inevitable. These uncertainties should be considered when calculating and reporting the average mass. Methods like standard deviation and error propagation can help quantify the uncertainty associated with the average mass value.
Non-Uniform Distributions: Beyond Simple Averages
For substances or datasets with non-uniform mass distributions, simple averages may not accurately represent the central tendency. More sophisticated statistical methods, such as median or mode, may be more appropriate in such cases.
Dealing with Extremely Large Datasets
For extremely large datasets, computational efficiency becomes a major consideration. Specialized algorithms and software tools might be necessary for efficient calculation of average mass.
Applications of Average Mass Calculation
The calculation of average mass finds applications in a wide range of fields:
- Chemistry: Determining the average molar mass of a mixture of isotopes, calculating the mass of reactants and products in chemical reactions.
- Physics: Calculating the center of mass of an object, analyzing the motion of particles in a system.
- Materials Science: Characterizing the properties of materials, analyzing the composition of alloys.
- Environmental Science: Measuring the average mass of pollutants in a sample, assessing the impact of environmental factors.
- Engineering: Designing structures, calculating the weight of components in a machine.
- Data Analysis and Statistics: Summarizing data, making comparisons across different groups.
Conclusion: A Practical Tool for Various Disciplines
Calculating average mass is a versatile and powerful tool with applications across numerous scientific, engineering, and statistical disciplines. Understanding the different methods for calculating average mass, along with their limitations and potential challenges, is crucial for accurate analysis and effective problem-solving. By employing the appropriate method and considering potential sources of error, researchers and analysts can confidently utilize average mass calculations to draw meaningful conclusions and make informed decisions. Remember to always choose the method that best suits your specific data and the nature of the problem you're trying to solve. This comprehensive guide provides a solid foundation for understanding and applying these fundamental concepts. Remember to always double-check your calculations and consider the context of your data for accurate and meaningful results.
Latest Posts
Latest Posts
-
A Hydrocarbon Is Any Compound That Contains
Mar 28, 2025
-
In Order To Break A Bond Energy Must Be
Mar 28, 2025
-
How Do You Divide A Square Root
Mar 28, 2025
-
Proof Of Derivative Of Inverse Trig Functions
Mar 28, 2025
-
Boundary Between The Crust And Mantle
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate The Average Mass . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
