Do Acids React With Metals To Produce Hydrogen Gas
Muz Play
Mar 17, 2025 · 6 min read
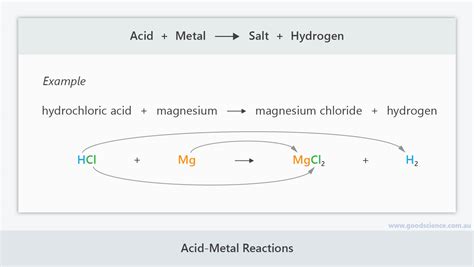
Table of Contents
Do Acids React with Metals to Produce Hydrogen Gas? A Comprehensive Guide
Acids reacting with metals to produce hydrogen gas is a fundamental concept in chemistry, with far-reaching implications in various fields. This reaction, a classic example of a single displacement reaction, is crucial for understanding corrosion, industrial processes, and even the development of new energy technologies. This comprehensive guide delves into the intricacies of this reaction, exploring the underlying principles, influencing factors, and practical applications.
Understanding the Reaction: A Single Displacement Affair
The reaction between an acid and a metal is a single displacement reaction, also known as a single replacement reaction. In this type of reaction, a more reactive metal displaces a less reactive element (usually hydrogen) from its compound. The general equation is:
Acid + Metal → Salt + Hydrogen gas
For example, the reaction between hydrochloric acid (HCl) and zinc (Zn) is:
2HCl(aq) + Zn(s) → ZnCl₂(aq) + H₂(g)
Here, zinc, being more reactive than hydrogen, displaces hydrogen from hydrochloric acid, forming zinc chloride (a salt) and hydrogen gas. The liberated hydrogen gas is easily observable as bubbles.
Factors Influencing the Reaction Rate
Several factors influence the rate at which this reaction proceeds:
1. Reactivity of the Metal: The reactivity series of metals dictates the likelihood and speed of the reaction. Highly reactive metals like alkali metals (e.g., sodium, potassium) react vigorously with acids, sometimes explosively. Less reactive metals like copper or gold don't react with many common acids. The higher the metal's position in the reactivity series, the faster the reaction.
2. Concentration of the Acid: A higher concentration of acid generally leads to a faster reaction rate. More acid molecules are available to collide with the metal surface, increasing the frequency of successful collisions and thus accelerating the reaction.
3. Surface Area of the Metal: A larger surface area of the metal exposes more metal atoms to the acid, leading to a faster reaction. Powdered metals react much faster than a solid chunk of the same metal because of the increased surface area.
4. Temperature: Increasing the temperature increases the kinetic energy of the reacting particles. This leads to more frequent and energetic collisions, resulting in a faster reaction rate.
5. Presence of Catalysts: Certain catalysts can speed up the reaction without being consumed themselves. These catalysts often facilitate the adsorption of reactants onto their surface, increasing the reaction rate.
Types of Acids and Their Reactions with Metals
Different acids react with metals at varying rates and produce different salts. Let's look at some common examples:
1. Strong Acids:**
Strong acids, like hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃), readily react with many metals. However, nitric acid's reactivity is complicated by the fact that it's a strong oxidizing agent. While it does react with metals to produce hydrogen, the hydrogen often gets oxidized further to water, resulting in the formation of nitrogen oxides instead of hydrogen gas.
-
Hydrochloric Acid (HCl): Reacts with most metals above hydrogen in the reactivity series, producing metal chlorides and hydrogen gas. For example:
- Fe(s) + 2HCl(aq) → FeCl₂(aq) + H₂(g) (Iron reacting with Hydrochloric Acid)
-
Sulfuric Acid (H₂SO₄): Reacts with many metals, forming metal sulfates and hydrogen gas. Concentrated sulfuric acid, however, exhibits oxidizing properties, particularly at higher temperatures, leading to the production of sulfur dioxide (SO₂) instead of hydrogen gas.
-
Nitric Acid (HNO₃): As mentioned earlier, its strong oxidizing nature often prevents the formation of significant amounts of hydrogen gas. Instead, it leads to the formation of nitrogen oxides and water, along with the corresponding metal nitrate salt.
2. Weak Acids:**
Weak acids, like acetic acid (CH₃COOH) and carbonic acid (H₂CO₃), react more slowly with metals compared to strong acids. The lower concentration of H⁺ ions in weak acids leads to a slower reaction rate.
-
Acetic Acid (CH₃COOH): Reacts slowly with some reactive metals, producing metal acetates and hydrogen gas. The reaction rate is significantly slower compared to strong acids.
-
Carbonic Acid (H₂CO₃): Though weak, it can still react with some very reactive metals, like alkali metals. However, this reaction is usually slower and less pronounced.
Practical Applications: From Industry to Everyday Life
The reaction of acids with metals to produce hydrogen gas has numerous applications across diverse fields:
1. Industrial Hydrogen Production:
The production of hydrogen gas through this reaction holds significant industrial importance. Many industrial processes utilize hydrogen as a raw material. Though other methods, like steam reforming, are dominant, the acid-metal reaction remains a viable option, particularly in smaller-scale applications and specific situations where pure hydrogen is required.
2. Metal Cleaning and Etching:**
This reaction is used in metal cleaning and etching processes. The reaction can remove oxides and other impurities from metal surfaces, preparing them for further processing or treatment.
3. Battery Technology:
Certain types of batteries, such as some fuel cells, rely on the reaction between acids and metals to generate electricity. The hydrogen gas produced can be used as a fuel source.
4. Laboratory Applications:**
The reaction is extensively used in chemistry laboratories to prepare and study hydrogen gas. It provides a simple and controlled method for generating small quantities of hydrogen in a laboratory setting.
5. Understanding Corrosion:**
This reaction is crucial for understanding corrosion, the gradual degradation of metals due to chemical reactions. The reaction of acids in the environment with metals leads to the formation of metal oxides and hydroxides, weakening the structural integrity of the metal.
Safety Precautions: Handling Acids and Hydrogen Gas
Hydrogen gas is highly flammable and can form explosive mixtures with air. Therefore, utmost care must be taken when working with acids and generating hydrogen gas. The following precautions are recommended:
-
Always wear appropriate safety gear: This includes safety goggles, gloves, and a lab coat.
-
Perform experiments in a well-ventilated area: To prevent the accumulation of hydrogen gas, which can lead to explosions.
-
Avoid open flames and sparks: Hydrogen gas is highly flammable.
-
Handle acids carefully: Avoid spills and skin contact.
-
Dispose of waste properly: Follow appropriate procedures for handling chemical waste.
Conclusion: A Fundamental Reaction with Broad Implications
The reaction between acids and metals to produce hydrogen gas is a fundamental chemical process with far-reaching implications. From industrial hydrogen production to understanding corrosion processes, this reaction is integral to many aspects of science, engineering, and technology. Understanding the underlying principles, influencing factors, and safety precautions associated with this reaction is crucial for anyone working in related fields. As we continue to explore new and sustainable energy solutions, this simple yet powerful reaction is likely to play an increasingly important role in the years to come. The continuous investigation and optimization of this reaction will further enhance its contribution to various sectors, solidifying its status as a cornerstone of chemical principles.
Latest Posts
Latest Posts
-
How Much Nadh Does Glycolysis Produce
Mar 17, 2025
-
What Is A Principal Agent In Insurance
Mar 17, 2025
-
Strong And Weak Acids And Bases List
Mar 17, 2025
-
Absorption Spectrum Of Helium Largest Transition
Mar 17, 2025
-
List Of Equipments For Validation In Pharma And Biotech Industry
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Do Acids React With Metals To Produce Hydrogen Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
