Do Covalent Compounds Dissolve In Water
Muz Play
Mar 18, 2025 · 6 min read
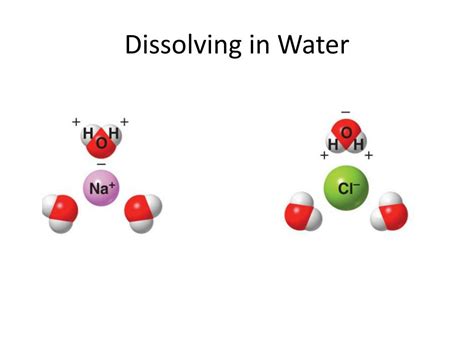
Table of Contents
Do Covalent Compounds Dissolve in Water? A Deep Dive into Solubility
The question of whether covalent compounds dissolve in water is a crucial one in chemistry, impacting numerous fields from biology to material science. The simple answer is: some do, some don't. Unlike the generally predictable behavior of ionic compounds (which tend to dissolve readily in water), the solubility of covalent compounds is far more nuanced and depends on several key factors. This article delves deep into the intricacies of covalent compound solubility, exploring the underlying principles, influencing factors, and practical implications.
Understanding the Nature of Covalent Bonds and Water
Before diving into the solubility of covalent compounds, it's crucial to establish a firm understanding of both covalent bonds and the unique properties of water.
Covalent Bonds: Sharing is Caring
Covalent bonds are formed when two or more atoms share electrons to achieve a stable electron configuration. This sharing creates a strong attraction between the atoms, holding them together to form molecules. Unlike ionic bonds, where electrons are completely transferred from one atom to another, covalent bonds involve a more equal distribution (though not always perfectly equal) of electrons. The strength of the covalent bond depends on several factors including the electronegativity difference between the atoms involved and the number of shared electron pairs. Examples of covalent compounds include methane (CH₄), glucose (C₆H₁₂O₆), and ethanol (C₂H₅OH).
Water: The Universal Solvent (But Not for Everyone)
Water (H₂O) is a unique and remarkable molecule. Its polar nature, stemming from the significant difference in electronegativity between oxygen and hydrogen, is key to its solvent properties. The oxygen atom is more electronegative, creating a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This creates a dipole moment, meaning the molecule possesses a positive and negative end, like a tiny magnet. This polarity allows water molecules to interact strongly with other polar molecules and ions.
The Solubility Dance: Factors Affecting Covalent Compound Dissolution
The solubility of a covalent compound in water is determined by the interplay of several factors:
1. Polarity: The Key Player
The most significant factor is polarity. Polar covalent compounds, those with a significant difference in electronegativity between atoms, resulting in a dipole moment, tend to dissolve in water. This is because the positive and negative ends of the water molecule can interact favorably with the positive and negative poles of the polar covalent molecule, a process called hydrogen bonding if the molecule contains O-H, N-H, or F-H bonds, and dipole-dipole interactions otherwise. This interaction overcomes the attractive forces within the covalent compound, allowing it to dissolve.
Examples of polar covalent compounds that dissolve in water include:
- Ethanol (C₂H₅OH): The hydroxyl group (-OH) makes it highly polar, facilitating hydrogen bonding with water.
- Glucose (C₆H₁₂O₆): The numerous hydroxyl groups contribute to its high polarity and solubility.
- Acetic acid (CH₃COOH): The carboxyl group (-COOH) provides significant polarity.
2. Molecular Size and Shape
Larger molecules, even if polar, may not dissolve readily in water due to the increased strength of the intermolecular forces within the molecule. Similarly, complex molecular shapes can hinder the interaction with water molecules, reducing solubility. Consider the difference between methanol (CH₃OH) and a long-chain alcohol like 1-octanol (CH₃(CH₂)₇OH). Methanol is highly soluble in water due to its small size, whereas 1-octanol's long hydrocarbon chain significantly reduces its solubility despite its polar hydroxyl group. The longer chain is primarily non-polar, and its interactions with water are outweighed by interactions between the hydrocarbon chains.
3. Hydrogen Bonding: A Powerful Force
The presence of hydrogen bonding between a covalent compound and water molecules significantly increases its solubility. The strength of hydrogen bonds allows for stronger interactions, helping to overcome the attractive forces between the covalent molecules themselves.
4. Temperature: A Temperature-Dependent Affair
Temperature often plays a crucial role in solubility. Increasing the temperature generally increases the kinetic energy of molecules, allowing for more effective interactions between the solute and solvent molecules and often leading to increased solubility. However, this is not always the case; some compounds exhibit decreased solubility with increased temperature.
5. Pressure: The Less Significant Factor
For most covalent compounds, the effect of pressure on solubility in water is minimal. However, under high pressure, the solubility of gases in water increases, significantly impacting the solubility of some gases that might otherwise form covalent interactions.
Covalent Compounds That Do Not Dissolve in Water: The Nonpolar Crew
Many covalent compounds are nonpolar, meaning their electrons are shared relatively equally between atoms, resulting in little to no dipole moment. These nonpolar compounds do not interact favorably with the polar water molecules. Instead, the attractive forces between water molecules are stronger than the interaction between the nonpolar molecule and water, hence preventing dissolution. This is summarized as "like dissolves like". Polar solvents dissolve polar solutes and non-polar solvents dissolve non-polar solutes.
Examples of nonpolar covalent compounds that are largely insoluble in water include:
- Hydrocarbons: Compounds composed solely of carbon and hydrogen (e.g., methane, hexane). The C-H bonds are largely nonpolar.
- Fats and oils: These are usually large molecules with long hydrocarbon chains, making them highly nonpolar.
- Many organic solvents: Compounds like benzene, toluene, and carbon tetrachloride are nonpolar and insoluble in water.
Practical Implications and Applications
The solubility of covalent compounds in water has far-reaching consequences in various fields:
1. Biology: Life's Watery Medium
Water is the solvent of life, and the solubility of biological molecules (many of which are covalent compounds) is crucial for biological processes. The solubility of sugars, amino acids, and other biomolecules is essential for their transport, metabolism, and interaction within cells. Conversely, the insolubility of certain lipids plays a crucial role in membrane structure and function.
2. Pharmaceuticals: Absorption and Delivery
The solubility of drugs (many of which are covalent compounds) determines their bioavailability—how effectively the body can absorb and utilize them. Poorly soluble drugs may be less effective because they are not readily absorbed into the bloodstream. Pharmaceutical scientists work extensively to modify drug molecules to enhance their water solubility, thereby improving their efficacy.
3. Environmental Science: Pollution and Remediation
The solubility of pollutants in water is critical in understanding their environmental impact and developing effective remediation strategies. For example, the solubility of oil and other hydrocarbons in water has significant implications for marine ecosystems and requires the development of effective techniques to remove the polluting substance from the water.
4. Industrial Chemistry: Reactions and Processes
Many industrial chemical processes rely on the solubility of covalent compounds in water. Dissolving reagents in water can facilitate reactions, and the solubility of products may be relevant to their separation and purification.
Conclusion: A Complex Relationship
The solubility of covalent compounds in water is not a simple yes or no answer. It is a complex phenomenon governed by the interplay of molecular polarity, size, shape, hydrogen bonding, temperature, and pressure. Understanding these factors is crucial for applications spanning biology, pharmaceuticals, environmental science, and industrial chemistry. The "like dissolves like" principle provides a useful guideline, but the specific behavior of a covalent compound in water often requires detailed analysis and consideration of its individual properties.
Latest Posts
Latest Posts
-
Final Product Of The Calvin Cycle
Mar 18, 2025
-
Is A Solution A Homogeneous Or Heterogeneous Mixture
Mar 18, 2025
-
Is S More Electronegative Than O
Mar 18, 2025
-
How Many Valence Electrons Are In Sulfur
Mar 18, 2025
-
What Is The Difference Between Volume Measurements And Capacities
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Do Covalent Compounds Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
