Does Adding A Catalyst Increase The Rate Of Reaction
Muz Play
Mar 26, 2025 · 5 min read
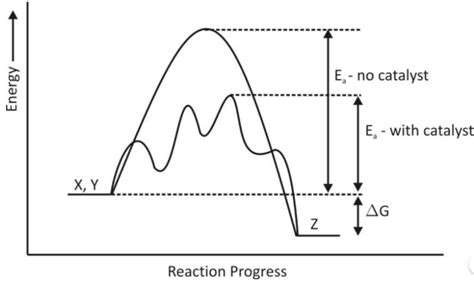
Table of Contents
- Does Adding A Catalyst Increase The Rate Of Reaction
- Table of Contents
- Does Adding a Catalyst Increase the Rate of Reaction? A Deep Dive into Catalysis
- Understanding Reaction Rates and Activation Energy
- How Catalysts Lower Activation Energy
- Types of Catalysts
- 1. Homogeneous Catalysts
- 2. Heterogeneous Catalysts
- Examples of Catalysts in Action
- Factors Affecting Catalyst Performance
- Conclusion: The Indispensable Role of Catalysts
- Latest Posts
- Latest Posts
- Related Post
Does Adding a Catalyst Increase the Rate of Reaction? A Deep Dive into Catalysis
The simple answer is a resounding yes. Adding a catalyst dramatically increases the rate of a chemical reaction without itself being consumed in the process. This seemingly magical ability underpins countless industrial processes, biological functions, and everyday phenomena. But understanding why catalysts work requires a deeper exploration of reaction mechanisms, activation energy, and the fascinating world of catalytic chemistry.
Understanding Reaction Rates and Activation Energy
Before delving into the role of catalysts, let's establish a foundational understanding of reaction rates and activation energy. Chemical reactions involve the breaking and forming of chemical bonds. The rate of reaction refers to how quickly reactants are converted into products. This rate is influenced by various factors, including:
- Concentration of reactants: Higher concentrations generally lead to faster reactions.
- Temperature: Increasing temperature provides molecules with more kinetic energy, increasing the frequency and success rate of collisions leading to reactions.
- Surface area (for heterogeneous reactions): A larger surface area increases the contact between reactants, enhancing the reaction rate.
- Presence of a catalyst: This is the core focus of our discussion.
The activation energy (Ea) represents the minimum energy required for a reaction to occur. Think of it as the energy barrier that reactant molecules must overcome to transform into products. Molecules need to collide with sufficient energy to break existing bonds and form new ones. A reaction with a high activation energy will proceed slowly, while one with a low activation energy will proceed quickly.
(Illustrative image of an energy diagram showing activation energy with and without a catalyst would be beneficial here. Imagine a graph with reaction coordinate on the x-axis and potential energy on the y-axis. Two curves, one higher representing the uncatalyzed reaction and a lower curve representing the catalyzed reaction, both showing the activation energy as the difference between the reactants' energy and the transition state's energy.)
How Catalysts Lower Activation Energy
The magic of a catalyst lies in its ability to lower the activation energy of a reaction. It does this by providing an alternative reaction pathway with a lower energy barrier. This doesn't change the overall energy difference between reactants and products (ΔH, enthalpy change), but it dramatically speeds up the reaction by making it easier for molecules to overcome the energy hurdle.
Catalysts achieve this lowering of activation energy through various mechanisms, including:
- Providing an alternative reaction pathway: The catalyst interacts with the reactants, forming intermediate complexes. These complexes then decompose to form the products and regenerate the catalyst. This new pathway has a lower activation energy than the uncatalyzed reaction.
- Orienting reactants: Catalysts can bring reactants into closer proximity and in the correct orientation for a successful reaction, increasing the probability of effective collisions.
- Weakening bonds: The interaction between the catalyst and reactants can weaken specific bonds in the reactants, making them easier to break and facilitating the reaction.
- Stabilizing transition states: The catalyst can stabilize the high-energy transition state, the point of highest energy along the reaction pathway, reducing the energy required to reach it.
Types of Catalysts
Catalysts are broadly classified into two main types:
1. Homogeneous Catalysts
Homogeneous catalysts exist in the same phase (solid, liquid, or gas) as the reactants. For example, the use of sulfuric acid (liquid) to catalyze the esterification of carboxylic acids (liquid) and alcohols (liquid) is a classic example of homogeneous catalysis. The acid protonates the carbonyl oxygen, making the carbon more electrophilic and more susceptible to nucleophilic attack by the alcohol.
2. Heterogeneous Catalysts
Heterogeneous catalysts exist in a different phase than the reactants. A common example is the use of platinum (solid) in the catalytic converter of a car to convert harmful gases (gaseous reactants) into less harmful products. In this case, the reaction occurs on the surface of the platinum catalyst. The reactants adsorb onto the surface, undergo reaction, and then desorb as products.
(Include an image comparing homogeneous and heterogeneous catalysis visually.)
Examples of Catalysts in Action
Catalysts are ubiquitous, playing crucial roles in various processes:
-
Industrial processes: The Haber-Bosch process, which produces ammonia from nitrogen and hydrogen, relies on an iron catalyst. The production of sulfuric acid, a cornerstone of the chemical industry, utilizes vanadium pentoxide as a catalyst. Many polymerization reactions, essential for the creation of plastics, also require catalysts.
-
Biological systems: Enzymes are biological catalysts, proteins that accelerate biochemical reactions within living organisms. They are highly specific, catalyzing only certain reactions. Examples include enzymes involved in digestion, respiration, and DNA replication. Enzymes work by binding to specific substrates and creating an environment conducive to reaction.
-
Everyday life: Catalytic converters in automobiles reduce harmful emissions. The decomposition of hydrogen peroxide into water and oxygen is often accelerated by manganese dioxide, a catalyst found in many cleaning products.
Factors Affecting Catalyst Performance
Several factors influence the effectiveness of a catalyst:
-
Temperature: While catalysts lower activation energy, increasing temperature often further enhances reaction rates by increasing the frequency of collisions. However, excessively high temperatures can damage or deactivate some catalysts.
-
Surface area (for heterogeneous catalysts): A larger surface area provides more active sites for the reaction to occur, enhancing the catalytic activity. This is why catalysts are often finely divided or porous.
-
Catalyst poisoning: Certain substances, known as poisons, can adsorb strongly onto the catalyst's active sites, blocking them and reducing catalytic activity. This is a significant concern in industrial applications.
-
Catalyst selectivity: A highly selective catalyst facilitates a specific reaction, minimizing the formation of unwanted byproducts.
-
Catalyst stability: A good catalyst should retain its activity and selectivity over time. However, catalysts can degrade due to factors such as sintering (the aggregation of catalyst particles) or poisoning.
Conclusion: The Indispensable Role of Catalysts
The addition of a catalyst significantly increases the rate of a chemical reaction by lowering its activation energy and providing an alternative reaction pathway. This fundamental principle underpins countless applications, from industrial processes to biological functions and everyday phenomena. Understanding the different types of catalysts, the factors affecting their performance, and the mechanisms by which they function is crucial for optimizing chemical reactions and driving advancements in various fields. The continued research and development of new and improved catalysts are essential for sustainable industrial practices and solving many of the world's challenges. The exploration of catalytic chemistry promises continued breakthroughs and innovations in the years to come. From minimizing environmental impact to developing more efficient energy sources, catalysts remain a critical component of technological advancements.
Latest Posts
Latest Posts
-
Dissolution Of Sodium Chloride In Water
Mar 30, 2025
-
What Is A Coefficient In Chemical Equations
Mar 30, 2025
-
Do Trailing Zeros Count As Sig Figs
Mar 30, 2025
-
How Many Valence Electrons Do Halogens Have
Mar 30, 2025
-
What Is The Modern Atomic Model
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Does Adding A Catalyst Increase The Rate Of Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
