How Many Valence Electrons Do Halogens Have
Muz Play
Mar 30, 2025 · 5 min read
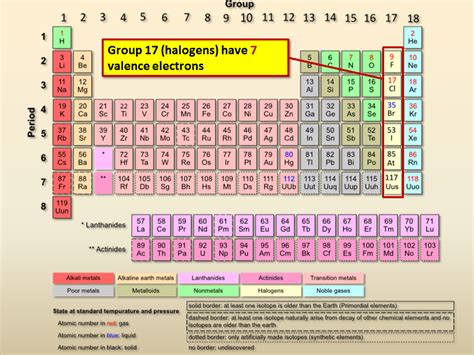
Table of Contents
How Many Valence Electrons Do Halogens Have? A Deep Dive into Group 17
Halogens, the fascinating elements of Group 17 on the periodic table, are known for their reactivity and diverse applications. Understanding their electronic structure, particularly the number of valence electrons, is key to comprehending their chemical behavior. This comprehensive guide will delve into the specifics of halogen valence electrons, exploring their electronic configuration, bonding characteristics, and the implications for their properties and reactivity.
Understanding Valence Electrons
Before focusing specifically on halogens, let's establish a foundational understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the most loosely held and, therefore, are primarily involved in chemical bonding. The number of valence electrons an atom possesses dictates its reactivity and the types of bonds it can form. Atoms tend to react in ways that achieve a stable electron configuration, often resembling that of a noble gas (Group 18 elements), which have a full outermost shell. This stability is often achieved through gaining, losing, or sharing electrons.
The Electronic Configuration of Halogens
Halogens (fluorine, chlorine, bromine, iodine, and astatine) are characterized by having seven valence electrons. This electronic configuration is what makes them highly reactive. Let's examine the electronic configuration of a few examples:
Fluorine (F)
- Atomic Number: 9
- Electronic Configuration: 1s²2s²2p⁵
- Valence Electrons: 7 (2s²2p⁵)
The two electrons in the 2s subshell and the five electrons in the 2p subshell constitute fluorine's seven valence electrons.
Chlorine (Cl)
- Atomic Number: 17
- Electronic Configuration: 1s²2s²2p⁶3s²3p⁵
- Valence Electrons: 7 (3s²3p⁵)
Chlorine's seven valence electrons reside in the third energy level (3s and 3p subshells).
Bromine (Br)
- Atomic Number: 35
- Electronic Configuration: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁵
- Valence Electrons: 7 (4s²4p⁵)
Iodine (I)
- Atomic Number: 53
- Electronic Configuration: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁵
- Valence Electrons: 7 (5s²5p⁵)
Astatine (At)
- Atomic Number: 85
- Electronic Configuration: 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s²4d¹⁰5p⁶6s²4f¹⁴5d¹⁰6p⁵
- Valence Electrons: 7 (6s²6p⁵)
As you can see, despite the increasing complexity of their electronic configurations as you move down the group, all halogens consistently exhibit seven valence electrons. This consistency is a defining characteristic of the group.
The Significance of Seven Valence Electrons
The presence of seven valence electrons profoundly impacts the chemical behavior of halogens. They are only one electron short of achieving the stable octet configuration of the noble gases. This drives their strong tendency to gain an electron, forming a stable negative ion (anion) with a -1 charge. This process is known as reduction.
Halogen Bonding and Reactivity
This drive to gain an electron makes halogens highly reactive, especially with metals which readily lose electrons (oxidation). The reactivity generally decreases as you move down the group. Fluorine is the most reactive halogen, followed by chlorine, bromine, iodine, and astatine. This trend is attributed to several factors, including atomic size and electronegativity.
Electronegativity
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Fluorine is the most electronegative element, meaning it strongly attracts electrons towards itself. This high electronegativity contributes significantly to its high reactivity.
Atomic Size
As you move down the group, the atomic size increases. This larger distance between the nucleus and the valence electrons means that the attraction between the nucleus and the incoming electron is weaker. Consequently, the reactivity decreases.
Ionic and Covalent Bonding
Halogens readily participate in both ionic and covalent bonding. In ionic bonding, they gain an electron from a metal, forming a stable ionic compound. For example, sodium chloride (NaCl), common table salt, is formed when chlorine gains an electron from sodium.
In covalent bonding, halogens share electrons with other nonmetals. For example, chlorine can form a covalent bond with another chlorine atom to form Cl₂, diatomic chlorine gas. In these covalent bonds, the halogens achieve a stable electron configuration through electron sharing.
Applications of Halogens
The unique properties stemming from their seven valence electrons make halogens crucial in various applications:
- Disinfection: Chlorine and other halogens are widely used as disinfectants in water treatment and sanitation. Their reactivity allows them to kill harmful bacteria and other microorganisms.
- Industrial Chemistry: Halogens are essential building blocks for various industrial chemicals, including refrigerants, plastics, and solvents.
- Medicine: Certain halogen compounds have important applications in medicine, including some anesthetics and antiseptics.
- Photography: Silver halides are crucial components in photographic films and papers.
Beyond the Basics: Exceptions and Nuances
While the seven valence electron rule is a reliable generalization for halogens, there are some nuances to consider:
- Excited States: Under specific conditions, like high temperatures or in the presence of strong electromagnetic radiation, a halogen atom could potentially promote one of its electrons to a higher energy level, temporarily altering the number of valence electrons in its excited state. However, this is a transient state and doesn't affect the fundamental behavior described above.
- Astatine's Radioactivity: Astatine, being radioactive, has a significantly shorter lifespan and more complex chemical behavior compared to the other halogens. Its reactivity is affected by its radioactivity, introducing additional factors beyond the basic seven valence electrons.
Conclusion
The seven valence electrons of halogens are the cornerstone of their remarkable chemical properties and reactivity. This configuration drives their tendency to gain an electron, forming stable -1 anions, leading to their extensive use in various applications. While subtle variations exist, particularly with astatine, the consistent presence of seven valence electrons remains a defining characteristic of Group 17 elements, emphasizing their unique and significant role in chemistry. Understanding this fundamental aspect of their electronic structure provides a crucial framework for comprehending their behavior and applications. The detailed exploration of their electronic configurations, bonding characteristics, and reactivity reinforces the importance of valence electrons in shaping the properties of elements and their compounds.
Latest Posts
Latest Posts
-
Is Hydrogen More Electronegative Than Oxygen
Apr 01, 2025
-
What Happens When An Atom Gains Or Loses An Electron
Apr 01, 2025
-
How To Name Ionic And Covalent Bonds
Apr 01, 2025
-
Liquid In A Liquid Solution Example
Apr 01, 2025
-
Express The Inequality Using Interval Notation
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Do Halogens Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
