Does He Have The Same Line Emission Spectrum As H
Muz Play
Mar 22, 2025 · 5 min read
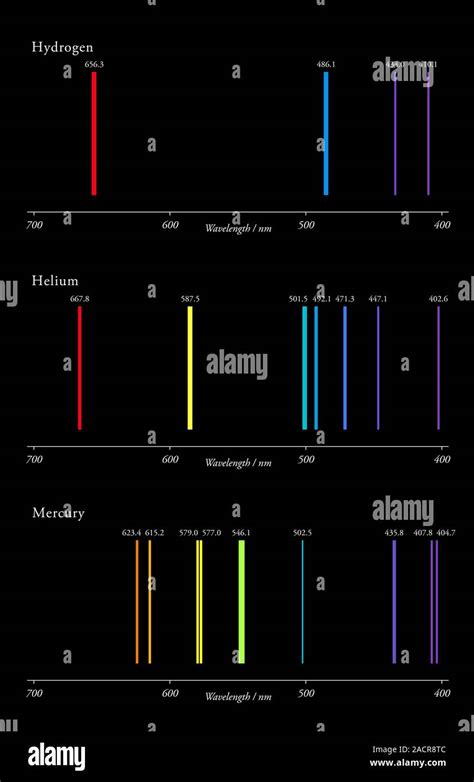
Table of Contents
Does He Have the Same Line Emission Spectrum as H? Understanding Atomic Fingerprints
The question, "Does he have the same line emission spectrum as H?" is inherently intriguing, but requires clarification. "He" likely refers to another element, and "H" almost certainly refers to hydrogen. The essence of the question delves into the fundamental principles of atomic structure and spectroscopy. The answer is a resounding no, except in highly specific and unlikely circumstances. Different elements possess unique emission spectra, acting like atomic fingerprints, and understanding why is crucial to comprehending the nature of matter and the universe.
Atomic Structure and Electron Transitions
At the heart of understanding emission spectra lies the Bohr model of the atom (though a more accurate quantum mechanical model is necessary for precision). This model depicts electrons orbiting the nucleus in specific energy levels or shells. These energy levels are quantized, meaning electrons can only exist at certain discrete energy states. They cannot exist between these levels.
When an electron absorbs energy (e.g., through heating or electrical discharge), it jumps to a higher energy level – an excited state. This state is unstable. The electron quickly returns to a lower energy level, releasing the absorbed energy as a photon of light. The energy of this photon is directly related to the energy difference between the two levels. Since these energy differences are unique to each element, the emitted light has a specific wavelength, resulting in a unique line emission spectrum.
Hydrogen's Unique Spectrum
Hydrogen (H), the simplest atom with one proton and one electron, exhibits a relatively straightforward emission spectrum. Its transitions between energy levels produce a series of distinct spectral lines, most notably the Lyman series (ultraviolet), Balmer series (visible), and Paschen series (infrared). The Balmer series, visible to the naked eye, is responsible for hydrogen's characteristic pink-red color in a flame test. The specific wavelengths of these lines are precisely calculable using the Rydberg formula.
The simplicity of hydrogen's spectrum stems from its single electron. The interactions and energy levels are easily modeled and predicted. More complex atoms, with multiple electrons, present a significantly more intricate situation.
The Complexity of Multi-Electron Atoms
Atoms with more than one electron have considerably more complex emission spectra. The presence of multiple electrons leads to several factors influencing the energy levels and transitions:
-
Electron-Electron Repulsion: Electrons repel each other, leading to deviations from the simple energy level predictions of the hydrogen atom. This repulsion modifies the energy levels, making precise calculations significantly more challenging.
-
Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus. This reduces the effective nuclear charge experienced by the outer electrons, affecting their energy levels and transitions.
-
Spin-Orbit Coupling: The interaction between the electron's spin and its orbital angular momentum further complicates the energy levels.
These factors result in a far richer and more complex emission spectrum than that of hydrogen. The number of spectral lines increases dramatically, with many more transitions possible. The wavelengths of these lines are unique to each element and are not easily predictable using simple formulas like the Rydberg equation.
Why Different Elements Have Different Spectra
The fundamental reason why different elements have different line emission spectra boils down to their unique nuclear charge and electron configurations. Each element has a unique number of protons in its nucleus (its atomic number), determining its positive charge. This charge interacts with its electrons, dictating their energy levels and the possible transitions between them. The arrangement of electrons in different energy levels and sublevels (electron configuration) further contributes to the complexity and uniqueness of each element’s spectral fingerprint.
This uniqueness is invaluable for various applications:
-
Elemental Analysis: Scientists use emission spectroscopy to identify the elemental composition of unknown samples. By analyzing the wavelengths of the emitted light, they can determine which elements are present.
-
Astronomy: Astronomers use spectroscopy to analyze the light from stars and other celestial objects. The emission spectra reveal the composition of these objects, providing crucial insights into their formation and evolution.
-
Forensic Science: Emission spectroscopy is used in forensic science to analyze trace evidence, helping to identify materials and substances involved in crimes.
Exceptional Cases: Ionized Atoms
While the vast majority of elements have unique and distinct spectra differing significantly from hydrogen, there is a specific scenario where a close resemblance could occur: highly ionized atoms. Consider a helium ion (He⁺), which has lost one electron. This ion is essentially a hydrogen-like atom, possessing only one electron orbiting the nucleus. Its emission spectrum would bear a resemblance to that of hydrogen, although the wavelengths would be shifted due to the larger nuclear charge of helium.
Similarly, other highly ionized atoms with only one remaining electron would produce spectra analogous to hydrogen's, but again, shifted in wavelength based on the nuclear charge. These are, however, exceptional cases and do not negate the general rule of unique spectral fingerprints for neutral atoms.
Conclusion
In conclusion, the answer to the question, "Does he have the same line emission spectrum as H?" is almost always no. Each element possesses a unique and characteristic emission spectrum, a consequence of its atomic structure and the interactions of its electrons. While highly ionized atoms with a single electron might show a resemblance to hydrogen's spectrum, the vast majority of elements exhibit profoundly different spectral fingerprints, making emission spectroscopy a powerful tool for identifying and analyzing matter. This principle is fundamental to our understanding of the universe, from the composition of distant stars to the analysis of materials here on Earth. The unique emission spectrum is, in essence, the atomic fingerprint that distinguishes one element from another.
Latest Posts
Latest Posts
-
Has A Definite Shape And Volume
Mar 23, 2025
-
How Is The Circulatory System Similar To A Road And Highway System
Mar 23, 2025
-
Diels Alder Reaction With Maleic Anhydride
Mar 23, 2025
-
Center Of Mass Of A Hemisphere
Mar 23, 2025
-
Organic Chemistry Substitution And Elimination Reactions
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Does He Have The Same Line Emission Spectrum As H . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
