Does Hydrogen Gain Or Lose Electrons
Muz Play
Mar 19, 2025 · 6 min read
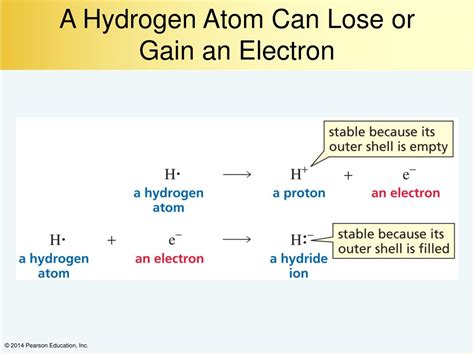
Table of Contents
Does Hydrogen Gain or Lose Electrons? Understanding Hydrogen's Reactivity
Hydrogen, the simplest and most abundant element in the universe, presents a fascinating case study in chemical bonding and reactivity. Its unique electronic structure dictates whether it gains or loses electrons, a crucial factor determining its role in various chemical reactions and its potential as a clean energy source. Unlike many elements with predictable behavior, hydrogen's electron gain or loss depends heavily on the specific chemical environment. This article will delve deep into the intricacies of hydrogen's reactivity, explaining the conditions under which it acts as an electron donor (losing electrons) or an electron acceptor (gaining electrons).
Hydrogen's Electronic Structure: The Foundation of Reactivity
Understanding hydrogen's behavior starts with its electronic structure. A neutral hydrogen atom possesses only one proton in its nucleus and one electron orbiting it. This single electron occupies the 1s atomic orbital, the orbital closest to the nucleus. This simple configuration is the key to hydrogen's versatility. Because it has only one electron, hydrogen can either lose this electron to achieve a stable empty shell (like helium), or gain an electron to achieve a stable full shell (also like helium). This duality is the reason behind hydrogen's ability to act as both an oxidizing and a reducing agent.
The Octet Rule and Hydrogen's Exception
The octet rule, a fundamental concept in chemistry, states that atoms tend to gain, lose, or share electrons in order to have eight electrons in their outermost shell (valence shell), achieving a stable electron configuration similar to noble gases. However, hydrogen is an exception. Its small size and only one electron mean it only needs two electrons in its valence shell to achieve the stable electron configuration of helium (1s²). This means hydrogen can achieve stability by either losing its single electron or gaining one.
Hydrogen as an Electron Donor: Formation of Cations (H⁺)
Hydrogen readily loses its single electron under specific circumstances, particularly when reacting with highly electronegative elements such as halogens (fluorine, chlorine, bromine, iodine) and oxygen. When hydrogen loses its electron, it forms a hydrogen cation (H⁺), also known as a proton. This process is typically observed in reactions with strong acids and bases, as well as in some redox reactions.
Examples of Hydrogen Losing Electrons:
-
Reaction with halogens: Hydrogen reacts with halogens to form hydrogen halides (e.g., HCl, HF, HBr, HI). In these reactions, hydrogen loses its electron to the halogen, which has a higher electronegativity. For instance, in the reaction between hydrogen and chlorine to form hydrogen chloride:
H₂ + Cl₂ → 2HCl
Here, each hydrogen atom loses an electron to a chlorine atom, forming H⁺ and Cl⁻ ions, which then attract each other electrostatically forming a covalent bond. Although the bond is covalent, the significant electronegativity difference between Hydrogen and Chlorine creates a polarized bond with a partial positive charge on Hydrogen and partial negative charge on Chlorine.
-
Reactions with strong acids: In strong acids like hydrochloric acid (HCl), hydrogen is bonded to a highly electronegative chlorine atom. In aqueous solution, the H-Cl bond is highly polarized, and the hydrogen atom essentially exists as a proton (H⁺) surrounded by water molecules, forming hydronium ions (H₃O⁺).
-
Redox reactions: Hydrogen can act as a reducing agent, donating electrons in redox reactions. In such cases, the hydrogen atom loses its electron, resulting in the formation of H⁺.
Hydrogen as an Electron Acceptor: Formation of Anions (H⁻)
Contrary to its tendency to lose electrons, hydrogen can also gain an electron under certain conditions. This results in the formation of a hydride anion (H⁻). This is observed when hydrogen reacts with electropositive elements, particularly alkali metals and alkaline earth metals. These metals readily lose electrons, providing the electron needed for hydrogen to achieve its stable helium-like configuration.
Examples of Hydrogen Gaining Electrons:
-
Reaction with alkali metals: Alkali metals, such as lithium (Li), sodium (Na), and potassium (K), are highly electropositive and readily lose electrons. When they react with hydrogen, they transfer an electron to hydrogen, forming a metal hydride. For example, the reaction between sodium and hydrogen forms sodium hydride:
2Na + H₂ → 2NaH
In this reaction, each sodium atom donates an electron to a hydrogen atom, resulting in the formation of Na⁺ cations and H⁻ anions. The resulting solid NaH contains ionic bonds.
-
Reaction with alkaline earth metals: Similar to alkali metals, alkaline earth metals like calcium (Ca) and magnesium (Mg) can also form hydrides:
Ca + H₂ → CaH₂
-
Formation of interstitial hydrides: Hydrogen can also occupy interstitial sites in the crystal lattice of transition metals, forming interstitial hydrides. These are usually non-stoichiometric compounds. In these cases, the electron transfer isn't entirely complete ionic behavior but exhibits more of a metallic characteristic.
Factors Influencing Hydrogen's Behavior: Electronegativity and Chemical Environment
The ultimate decision of whether hydrogen will gain or lose an electron is heavily influenced by the electronegativity of the element it interacts with, as well as the overall chemical environment.
Electronegativity: The Key Determinant
Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. When hydrogen reacts with an element of significantly higher electronegativity (like oxygen or chlorine), it acts as an electron donor (losing electrons). Conversely, when it reacts with an element of significantly lower electronegativity (like sodium or lithium), it acts as an electron acceptor (gaining electrons).
Chemical Environment: A Complicating Factor
The chemical environment surrounding the hydrogen atom also plays a significant role in determining its behavior. Factors like temperature, pressure, and the presence of catalysts can influence the reaction pathway and consequently whether hydrogen acts as a donor or acceptor.
Hydrogen's Role in Different Chemical Processes
Hydrogen's ability to both gain and lose electrons makes it a crucial element in a vast array of chemical processes. Its versatility allows it to participate in diverse reactions, from simple acid-base reactions to complex redox processes.
Hydrogen in Acid-Base Chemistry
Hydrogen plays a central role in acid-base chemistry. In Arrhenius and Brønsted-Lowry acid-base theories, acids are defined as substances that donate protons (H⁺), while bases are substances that accept protons. This highlights hydrogen's role as a proton donor in acidic solutions.
Hydrogen in Redox Reactions
Hydrogen’s role in redox reactions is equally significant. It can act both as an oxidizing agent (gaining electrons) and a reducing agent (losing electrons), depending on the specific reaction. Its ability to readily donate or accept electrons makes it a versatile component in many redox processes, often used in industrial applications.
Hydrogen as a Fuel Source: Clean Energy Implications
The increasing interest in hydrogen as a clean energy source stems from its ability to participate in combustion reactions that produce only water as a byproduct, making it an environmentally friendly fuel. In fuel cells, hydrogen undergoes oxidation at the anode, releasing electrons that flow through an external circuit to generate electricity. This process highlights the electron donation capability of hydrogen.
Conclusion: Hydrogen's Versatility in Chemical Reactions
Hydrogen's unique electronic structure allows it to function as both an electron donor and an electron acceptor, making it a remarkably versatile element in the chemical world. Whether it gains or loses electrons depends critically on the electronegativity of the atom it interacts with and the surrounding chemical environment. Understanding this duality is key to comprehending hydrogen's vital role in various chemical processes, from simple acid-base reactions to complex redox reactions, and ultimately its potential as a clean and sustainable energy source. Further research and advancements in this area could significantly impact our understanding of hydrogen's behavior and its potential applications in various fields.
Latest Posts
Latest Posts
-
Is Naoh A Base Or Acid
Mar 19, 2025
-
Confidence Interval When Standard Deviation Is Unknown
Mar 19, 2025
-
A Rate Of Change Velocity Or Acceleration
Mar 19, 2025
-
How Is Specific Gravity Related To Density
Mar 19, 2025
-
What Is The Difference Between Heat Capacity And Specific Heat
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Does Hydrogen Gain Or Lose Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
